Tau accumulation is associated with dopamine deficiency in vivo in four-repeat tauopathies
- PMID: 38366196
- PMCID: PMC11139736
- DOI: 10.1007/s00259-024-06637-6
Tau accumulation is associated with dopamine deficiency in vivo in four-repeat tauopathies
Abstract
Purpose: We hypothesized that severe tau burden in brain regions involved in direct or indirect pathways of the basal ganglia correlate with more severe striatal dopamine deficiency in four-repeat (4R) tauopathies. Therefore, we correlated [18F]PI-2620 tau-positron-emission-tomography (PET) imaging with [123I]-Ioflupane single-photon-emission-computed tomography (SPECT) for dopamine transporter (DaT) availability.
Methods: Thirty-eight patients with clinically diagnosed 4R-tauopathies (21 male; 69.0 ± 8.5 years) and 15 patients with clinically diagnosed α-synucleinopathies (8 male; 66.1 ± 10.3 years) who underwent [18F]PI-2620 tau-PET and DaT-SPECT imaging with a time gap of 3 ± 5 months were evaluated. Regional Tau-PET signals and DaT availability as well as their principal components were correlated in patients with 4R-tauopathies and α-synucleinopathies. Both biomarkers and the residuals of their association were correlated with clinical severity scores in 4R-tauopathies.
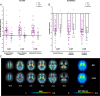
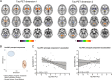
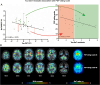
Results: In patients with 4R-tauopathies, [18F]PI-2620 binding in basal ganglia and midbrain regions was negatively associated with striatal DaT availability (i.e. globus pallidus internus and putamen (β = - 0.464, p = 0.006, Durbin-Watson statistics = 1.824) in a multiple regression model. Contrarily, [18F]PI-2620 binding in the dentate nucleus showed no significant regression factor with DaT availability in the striatum (β = 0.078, p = 0.662, Durbin-Watson statistics = 1.686). Patients with α-synucleinopathies did not indicate any regional associations between [18F]PI-2620-binding and DaT availability. Higher DaT-SPECT binding relative to tau burden was associated with better clinical performance (β = - 0.522, p = 0.011, Durbin-Watson statistics = 2.663) in patients with 4R-tauopathies.
Conclusion: Tau burden in brain regions involved in dopaminergic pathways is associated with aggravated dopaminergic dysfunction in patients with clinically diagnosed primary tauopathies. The ability to sustain dopamine transmission despite tau accumulation may preserve motor function.
Keywords: 4R-Tau; DaT imaging; Motor reserve; [18F]PI-2620 tau-PET.
© 2024. The Author(s).
Conflict of interest statement
Christian Ferschmann: none
Konstantin Messerschmidt: reader consultant honorarium from Life Molecular Imaging GmbH, 13353 Berlin, Germany
Johannes Gnörich: reader honorarium from Life Molecular Imaging GmbH, 13353 Berlin, Germany
Henryk Barthel: reader consultant honorarium from Life Molecular Imaging GmbH, 13353 Berlin, Germany
Ken Marek: none
Carla Palleis: none
Sabrina Katzdobler: none
Anna Stockbauer: none
Urban Fietzek: none
Anika Finze: none
Gloria Biechele: none
Leonie Beyer: none
Florian Eckenweber: none
Stephan Wall: none
Dorothee Saur: none
Matthias L. Schroeter: none
Jost-Julian Rumpf: none
Michael Rullmann: none
Andreas Schildan: none
Marianne Patt: none
Andrew Stephens: employee of Life Molecular Imaging
Joseph Classen: none
Peter Bartenstein: none
John Seibyl: none
Nicolai Franzmeier: none
Johannes Levin: reports speaker fees from Bayer Vital, Biogen, EISAI, TEVA and Roche, consulting fees from Axon Neuroscience and Biogen, author fees from Thieme medical publishers and W. Kohlhammer GmbH medical publishers and is inventor in a patent “Oral Phenylbutyrate for Treatment of Human 4-Repeat Tauopathies” (EP 23 156 122.6) filed by LMU Munich. In addition, he reports compensation for serving as chief medical officer for MODAG GmbH, is beneficiary of the phantom share program of MODAG GmbH and is inventor in a patent “Pharmaceutical Composition and Methods of Use” (EP 22 159 408.8) filed by MODAG GmbH, all activities outside the submitted work
Günter U. Höglinger: none related to this work
Osama Sabri: received research support from Life Molecular Imaging
Matthias Brendel: received speaker honoraria from GE healthcare, Roche and Life Molecular Imaging (LMI) and is an advisor of LMI
Maximilian Scheifele: reader honorarium from Life Molecular Imaging GmbH, 13353 Berlin, Germany
Figures


Similar articles
-
Subcortical tau is linked to hypoperfusion in connected cortical regions in 4-repeat tauopathies.Brain. 2024 Jul 5;147(7):2428-2439. doi: 10.1093/brain/awae174. Brain. 2024. PMID: 38842726
-
Neuroinflammation Parallels 18F-PI-2620 Positron Emission Tomography Patterns in Primary 4-Repeat Tauopathies.Mov Disord. 2024 Sep;39(9):1480-1492. doi: 10.1002/mds.29924. Epub 2024 Jul 18. Mov Disord. 2024. PMID: 39022835
-
Pragmatic algorithm for visual assessment of 4-Repeat tauopathies in [18F]PI-2620 PET Scans.Neuroimage. 2025 Feb 1;306:121001. doi: 10.1016/j.neuroimage.2025.121001. Epub 2025 Jan 9. Neuroimage. 2025. PMID: 39798829
-
Tau PET imaging in neurodegenerative tauopathies-still a challenge.Mol Psychiatry. 2019 Aug;24(8):1112-1134. doi: 10.1038/s41380-018-0342-8. Epub 2019 Jan 11. Mol Psychiatry. 2019. PMID: 30635637 Free PMC article. Review.
-
PE2I: a radiopharmaceutical for in vivo exploration of the dopamine transporter.CNS Neurosci Ther. 2008 Spring;14(1):47-64. doi: 10.1111/j.1527-3458.2007.00033.x. CNS Neurosci Ther. 2008. PMID: 18482099 Free PMC article. Review.
Cited by
-
Identification of metabolic progression and subtypes in progressive supranuclear palsy by PET molecular imaging.Eur J Nucl Med Mol Imaging. 2025 Feb;52(3):823-835. doi: 10.1007/s00259-024-06954-w. Epub 2024 Oct 23. Eur J Nucl Med Mol Imaging. 2025. PMID: 39438298
-
The Spectrum of Cognitive Impairment in Atypical Parkinsonism Syndromes: A Comprehensive Review of Current Understanding and Research.Diseases. 2025 Jan 31;13(2):39. doi: 10.3390/diseases13020039. Diseases. 2025. PMID: 39997046 Free PMC article. Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

