Inter-plasmid transfer of antibiotic resistance genes accelerates antibiotic resistance in bacterial pathogens
- PMID: 38366209
- PMCID: PMC10881300
- DOI: 10.1093/ismejo/wrad032
Inter-plasmid transfer of antibiotic resistance genes accelerates antibiotic resistance in bacterial pathogens
Abstract
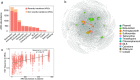
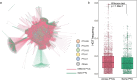
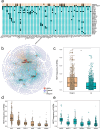
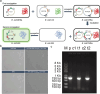
Antimicrobial resistance is a major threat for public health. Plasmids play a critical role in the spread of antimicrobial resistance via horizontal gene transfer between bacterial species. However, it remains unclear how plasmids originally recruit and assemble various antibiotic resistance genes (ARGs). Here, we track ARG recruitment and assembly in clinically relevant plasmids by combining a systematic analysis of 2420 complete plasmid genomes and experimental validation. Results showed that ARG transfer across plasmids is prevalent, and 87% ARGs were observed to potentially transfer among various plasmids among 8229 plasmid-borne ARGs. Interestingly, recruitment and assembly of ARGs occur mostly among compatible plasmids within the same bacterial cell, with over 88% of ARG transfers occurring between compatible plasmids. Integron and insertion sequences drive the ongoing ARG acquisition by plasmids, especially in which IS26 facilitates 63.1% of ARG transfer events among plasmids. In vitro experiment validated the important role of IS26 involved in transferring gentamicin resistance gene aacC1 between compatible plasmids. Network analysis showed four beta-lactam genes (blaTEM-1, blaNDM-4, blaKPC-2, and blaSHV-1) shuffling among 1029 plasmids and 45 clinical pathogens, suggesting that clinically alarming ARGs transferred accelerate the propagation of antibiotic resistance in clinical pathogens. ARGs in plasmids are also able to transmit across clinical and environmental boundaries, in terms of the high-sequence similarities of plasmid-borne ARGs between clinical and environmental plasmids. This study demonstrated that inter-plasmid ARG transfer is a universal mechanism for plasmid to recruit various ARGs, thus advancing our understanding of the emergence of multidrug-resistant plasmids.
Keywords: ESKAPE pathogens; antibiotic resistance genes; antimicrobial resistance; bioinformatics; horizontal gene transfer; plasmids.
© The Author(s) 2024. Published by Oxford University Press on behalf of the International Society for Microbial Ecology.
Conflict of interest statement
None declared.
Figures

Similar articles
-
Horizontal plasmid transfer promotes antibiotic resistance in selected bacteria in Chinese frog farms.Environ Int. 2024 Aug;190:108905. doi: 10.1016/j.envint.2024.108905. Epub 2024 Jul 23. Environ Int. 2024. PMID: 39089095
-
Tracking of Antibiotic Resistance Transfer and Rapid Plasmid Evolution in a Hospital Setting by Nanopore Sequencing.mSphere. 2020 Aug 19;5(4):e00525-20. doi: 10.1128/mSphere.00525-20. mSphere. 2020. PMID: 32817379 Free PMC article.
-
Cryptic environmental conjugative plasmid recruits a novel hybrid transposon resulting in a new plasmid with higher dispersion potential.mSphere. 2024 Jun 25;9(6):e0025224. doi: 10.1128/msphere.00252-24. Epub 2024 May 21. mSphere. 2024. PMID: 38771049 Free PMC article.
-
Plasmid-mediated antibiotic resistance gene transfer under environmental stresses: Insights from laboratory-based studies.Sci Total Environ. 2023 Aug 20;887:163870. doi: 10.1016/j.scitotenv.2023.163870. Epub 2023 May 5. Sci Total Environ. 2023. PMID: 37149187 Review.
-
A systematic review and meta-analysis on antibiotic resistance genes in Ghana.BMC Med Genomics. 2025 Mar 12;18(1):47. doi: 10.1186/s12920-024-02050-y. BMC Med Genomics. 2025. PMID: 40075357 Free PMC article.
Cited by
-
Regional antimicrobial resistance gene flow among the One Health sectors in China.Microbiome. 2025 Jan 7;13(1):3. doi: 10.1186/s40168-024-01983-x. Microbiome. 2025. PMID: 39763003 Free PMC article.
-
Secreted Expression of Thymosin β4 from Pinctada fucata in Pichia pastoris and Its Biological Activity.Biology (Basel). 2025 May 15;14(5):553. doi: 10.3390/biology14050553. Biology (Basel). 2025. PMID: 40427742 Free PMC article.
-
Clonal and horizontal transmission of carbapenem-resistant Enterobacterales strains and genes via flies.Gut Pathog. 2024 Nov 16;16(1):70. doi: 10.1186/s13099-024-00665-1. Gut Pathog. 2024. PMID: 39550588 Free PMC article.
-
Carbapenem-Resistant Pseudomonas aeruginosa's Resistome: Pan-Genomic Plasticity, the Impact of Transposable Elements and Jumping Genes.Antibiotics (Basel). 2025 Mar 31;14(4):353. doi: 10.3390/antibiotics14040353. Antibiotics (Basel). 2025. PMID: 40298491 Free PMC article. Review.
-
The extended mobility of plasmids.Nucleic Acids Res. 2025 Jul 19;53(14):gkaf652. doi: 10.1093/nar/gkaf652. Nucleic Acids Res. 2025. PMID: 40694848 Free PMC article. Review.
References
-
- Arcari G, Di Lella FM, Bibbolino Get al. . A multispecies cluster of vim-1 carbapenemase-producing enterobacterales linked by a novel, highly conjugative, and broad-host-range Inca plasmid forebodes the reemergence of vim-1. Antimicrob Agents Ch. 2020;64:64. 10.1128/AAC.02435-19. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

