Multiscale biochemical mapping of the brain through deep-learning-enhanced high-throughput mass spectrometry
- PMID: 38366241
- PMCID: PMC10927565
- DOI: 10.1038/s41592-024-02171-3
Multiscale biochemical mapping of the brain through deep-learning-enhanced high-throughput mass spectrometry
Abstract
Spatial omics technologies can reveal the molecular intricacy of the brain. While mass spectrometry imaging (MSI) provides spatial localization of compounds, comprehensive biochemical profiling at a brain-wide scale in three dimensions by MSI with single-cell resolution has not been achieved. We demonstrate complementary brain-wide and single-cell biochemical mapping using MEISTER, an integrative experimental and computational mass spectrometry (MS) framework. Our framework integrates a deep-learning-based reconstruction that accelerates high-mass-resolving MS by 15-fold, multimodal registration creating three-dimensional (3D) molecular distributions and a data integration method fitting cell-specific mass spectra to 3D datasets. We imaged detailed lipid profiles in tissues with millions of pixels and in large single-cell populations acquired from the rat brain. We identified region-specific lipid contents and cell-specific localizations of lipids depending on both cell subpopulations and anatomical origins of the cells. Our workflow establishes a blueprint for future development of multiscale technologies for biochemical characterization of the brain.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures








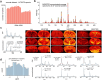
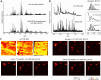



Update of
-
Integrative Multiscale Biochemical Mapping of the Brain via Deep-Learning-Enhanced High-Throughput Mass Spectrometry.bioRxiv [Preprint]. 2023 Nov 18:2023.05.31.543144. doi: 10.1101/2023.05.31.543144. bioRxiv. 2023. Update in: Nat Methods. 2024 Mar;21(3):521-530. doi: 10.1038/s41592-024-02171-3. PMID: 37398021 Free PMC article. Updated. Preprint.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

