The vomeronasal system of the wolf (Canis lupus signatus): The singularities of a wild canid
- PMID: 38366249
- PMCID: PMC11161832
- DOI: 10.1111/joa.14024
The vomeronasal system of the wolf (Canis lupus signatus): The singularities of a wild canid
Abstract
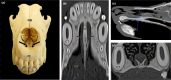
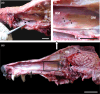
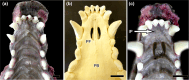
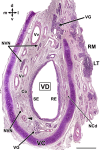
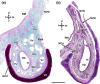
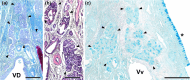
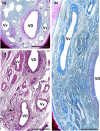
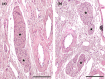
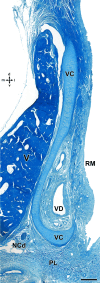
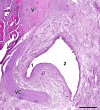
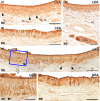
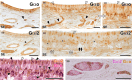
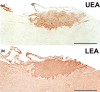
Wolves, akin to their fellow canids, extensively employ chemical signals for various aspects of communication, including territory maintenance, reproductive synchronisation and social hierarchy signalling. Pheromone-mediated chemical communication operates unconsciously among individuals, serving as an innate sensory modality that regulates both their physiology and behaviour. Despite its crucial role in the life of the wolf, there is a lacuna in comprehensive research on the neuroanatomical and physiological underpinnings of chemical communication within this species. This study investigates the vomeronasal system (VNS) of the Iberian wolf, simultaneously probing potential alterations brought about by dog domestication. Our findings demonstrate the presence of a fully functional VNS, vital for pheromone-mediated communication, in the Iberian wolf. While macroscopic similarities between the VNS of the wolf and the domestic dog are discernible, notable microscopic differences emerge. These distinctions include the presence of neuronal clusters associated with the sensory epithelium of the vomeronasal organ (VNO) and a heightened degree of differentiation of the accessory olfactory bulb (AOB). Immunohistochemical analyses reveal the expression of the two primary families of vomeronasal receptors (V1R and V2R) within the VNO. However, only the V1R family is expressed in the AOB. These findings not only yield profound insights into the VNS of the wolf but also hint at how domestication might have altered neural configurations that underpin species-specific behaviours. This understanding holds implications for the development of innovative strategies, such as the application of semiochemicals for wolf population management, aligning with contemporary conservation goals.
Keywords: accessory olfactory bulb; chemical communication; immunohistochemistry; lectins; pheromones; vomeronasal organ; vomeronasal system; wolf.
© 2024 The Authors. Journal of Anatomy published by John Wiley & Sons Ltd on behalf of Anatomical Society.
Figures








References
-
- Abellán‐Álvaro, M. , Martínez‐García, F. , Lanuza, E. & Agustín‐Pavón, C. (2022) Inhibition of the medial amygdala disrupts escalated aggression in lactating female mice after repeated exposure to male intruders. Communications Biology, 5(1), 980. Available from: 10.1038/s42003-022-03928-2 - DOI - PMC - PubMed
-
- Alonso, J.R. , Briñón, J.G. , Crespo, C. , Bravo, I.G. , Arévalo, R. & Aijón, J. (2001) Chemical organization of the macaque monkey olfactory bulb: II. Calretinin, calbindin D‐28k, parvalbumin, and neurocalcin immunoreactivity: calcium‐binding proteins in the monkey OB. Journal of Comparative Neurology, 432(3), 389–407. Available from: 10.1002/cne.1110 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

