Preclinical evaluation of new GRPR-antagonists with improved metabolic stability for radiotheranostic use in oncology
- PMID: 38366299
- PMCID: PMC10873254
- DOI: 10.1186/s41181-024-00242-6
Preclinical evaluation of new GRPR-antagonists with improved metabolic stability for radiotheranostic use in oncology
Abstract
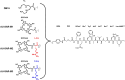
Background: The gastrin-releasing peptide receptor (GRPR) has been extensively studied as a biomolecular target for peptide-based radiotheranostics. However, the lack of metabolic stability and the rapid clearance of peptide radioligands, including radiolabeled GRPR-antagonists, often impede clinical application. Aiming at circumventing these drawbacks, we have designed three new GRPR-antagonist radioligands using [99mTc]Tc-DB15 ([99mTc]Tc-N4-AMA-DIG-DPhe-Gln-Trp-Ala-Val-Sar-His-Leu-NHEt; AMA: p-aminomethylaniline; DIG: diglycolate) as a motif, due to its high GRPR-affinity and stability to neprilysin (NEP). The new analogues carry the DOTAGA-chelator (1,4,7,10-tetraazacyclododecane-1-glutaric acid-4,7,10-triacetic acid) through different linkers at the N-terminus to allow for labeling with the theranostic radionuclide pair In-111/Lu-177. After labeling with In-111 the following radioligands were evaluated: (i) [111In]In-AU-SAR-M1 ([111In]In-DOTAGA-AMA-DIG-DPhe-Gln-Trp-Ala-Val-Sar-His-Leu-NHEt), (ii) [111In]In-AU-SAR-M2 ([111In]In-[DOTAGA-Arg]AU-SAR-M1) and (iii) [111In]In-AU-SAR-M3 ([111In]In-[DOTAGA-DArg]AU-SAR-M1).
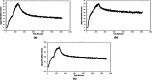
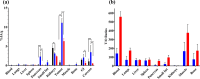
Results: These radioligands were compared in a series of in vitro assays using prostate adenocarcinoma PC-3 cells and in murine models. They all displayed high and GRPR-specific uptake in PC-3 cells. Analysis of mice blood collected 5 min post-injection (pi) revealed similar or even higher metabolic stability of the new radioligands compared with [99mTc]Tc-DB15. The stability could be further increased when the mice were treated with Entresto® to in situ induce NEP-inhibition. In PC-3 xenograft-bearing mice, [111In]In-AU-SAR-M1 displayed the most favourable biodistribution profile, combining a good tumor retention with the highest tumor-to-organ ratios, with the kidneys as the dose-limiting organ.
Conclusions: These findings strongly point at AU-SAR-M1 as a promising radiotherapeutic candidate when labeled with Lu-177, or other medically appealing therapeutic radiometals, especially when combined with in situ NEP-inhibition. To this goal further investigations are currently pursued.
Keywords: GRPR-antagonist; Metabolic stability; NEP-inhibition; PC-3 tumors; Prostate cancer; Radiotheranostics.
© 2024. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures





References
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
