In Staphylococcus aureus, the acyl-CoA synthetase MbcS supports branched-chain fatty acid synthesis from carboxylic acid and aldehyde precursors
- PMID: 38366323
- PMCID: PMC11167679
- DOI: 10.1111/mmi.15237
In Staphylococcus aureus, the acyl-CoA synthetase MbcS supports branched-chain fatty acid synthesis from carboxylic acid and aldehyde precursors
Abstract
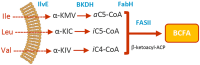
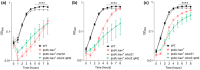
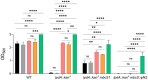
In the human pathogen Staphylococcus aureus, branched-chain fatty acids (BCFAs) are the most abundant fatty acids in membrane phospholipids. Strains deficient for BCFAs synthesis experience auxotrophy in laboratory culture and attenuated virulence during infection. Furthermore, the membrane of S. aureus is among the main targets for antibiotic therapy. Therefore, determining the mechanisms involved in BCFAs synthesis is critical to manage S. aureus infections. Here, we report that the overexpression of SAUSA300_2542 (annotated to encode an acyl-CoA synthetase) restores BCFAs synthesis in strains lacking the canonical biosynthetic pathway catalyzed by the branched-chain α-keto acid dehydrogenase (BKDH) complex. We demonstrate that the acyl-CoA synthetase activity of MbcS activates branched-chain carboxylic acids (BCCAs), and is required by S. aureus to utilize the isoleucine derivative 2-methylbutyraldehyde to restore BCFAs synthesis in S. aureus. Based on the ability of some staphylococci to convert branched-chain aldehydes into their respective BCCAs and our findings demonstrating that branched-chain aldehydes are in fact BCFAs precursors, we propose that MbcS promotes the scavenging of exogenous BCCAs and mediates BCFA synthesis via a de novo alternative pathway.
Keywords: Staphylococcus aureus; BKDH complex; MRSA; branched‐chain fatty acids; fatty acids metabolism; membrane phospholipids.
© 2024 The Authors. Molecular Microbiology published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare that there are no conflicts of interest.
Figures





Similar articles
-
A short-chain acyl-CoA synthetase that supports branched-chain fatty acid synthesis in Staphylococcus aureus.J Biol Chem. 2023 Apr;299(4):103036. doi: 10.1016/j.jbc.2023.103036. Epub 2023 Feb 16. J Biol Chem. 2023. PMID: 36806679 Free PMC article.
-
Roles of pyruvate dehydrogenase and branched-chain α-keto acid dehydrogenase in branched-chain membrane fatty acid levels and associated functions in Staphylococcus aureus.J Med Microbiol. 2018 Apr;67(4):570-578. doi: 10.1099/jmm.0.000707. Epub 2018 Mar 2. J Med Microbiol. 2018. PMID: 29498620 Free PMC article.
-
Regulation of the Sae Two-Component System by Branched-Chain Fatty Acids in Staphylococcus aureus.mBio. 2022 Oct 26;13(5):e0147222. doi: 10.1128/mbio.01472-22. Epub 2022 Sep 22. mBio. 2022. PMID: 36135382 Free PMC article.
-
Peroxisomal lipid degradation via beta- and alpha-oxidation in mammals.Cell Biochem Biophys. 2000;32 Spring:73-87. doi: 10.1385/cbb:32:1-3:73. Cell Biochem Biophys. 2000. PMID: 11330072 Review.
-
Role of hepatic fatty acid:coenzyme A ligases in the metabolism of xenobiotic carboxylic acids.Clin Exp Pharmacol Physiol. 1998 Oct;25(10):776-82. doi: 10.1111/j.1440-1681.1998.tb02152.x. Clin Exp Pharmacol Physiol. 1998. PMID: 9784915 Review.
Cited by
-
CodY controls the SaeR/S two-component system by modulating branched-chain fatty acid synthesis in Staphylococcus aureus.J Bacteriol. 2024 Nov 21;206(11):e0019124. doi: 10.1128/jb.00191-24. Epub 2024 Oct 9. J Bacteriol. 2024. PMID: 39382300 Free PMC article.
-
Pyrimidine sufficiency is required for Sae two-component system signaling in Staphylococcus aureus.bioRxiv [Preprint]. 2025 Mar 20:2025.03.20.644390. doi: 10.1101/2025.03.20.644390. bioRxiv. 2025. Update in: J Bacteriol. 2025 Aug 21;207(8):e0011525. doi: 10.1128/jb.00115-25. PMID: 40166268 Free PMC article. Updated. Preprint.
-
Fatty acid synthesis and utilization in gram-positive bacteria: insights from Bacillus subtilis.Microbiol Mol Biol Rev. 2025 Jun 25;89(2):e0006923. doi: 10.1128/mmbr.00069-23. Epub 2025 May 28. Microbiol Mol Biol Rev. 2025. PMID: 40434073 Review.
-
Pyrimidine sufficiency is required for Sae two-component system signaling in Staphylococcus aureus.J Bacteriol. 2025 Aug 21;207(8):e0011525. doi: 10.1128/jb.00115-25. Epub 2025 Jul 21. J Bacteriol. 2025. PMID: 40689635 Free PMC article.
References
-
- Barrick, J.E. , Yu, D.S. , Yoon, S.H. , Jeong, H. , Oh, T.K. , Schneider, D. et al. (2009) Genome evolution and adaptation in a long‐term experiment with Escherichia coli . Nature, 461, 1243–1247. - PubMed
-
- Beck, H.C. (2005) Branched‐chain fatty acid biosynthesis in a branched‐chain amino acid aminotransferase mutant of Staphylococcus carnosus . FEMS Microbiology Letters, 243, 37–44. - PubMed
-
- Beck, H.C. , Hansen, A.M. & Lauritsen, F.R. (2002) Metabolite production and kinetics of branched‐chain aldehyde oxidation in Staphylococcus xylosus . Enzyme and Microbial Technology, 31(1), 94–101.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

