Far-red light-enhanced apical dominance stimulates flower and fruit abortion in sweet pepper
- PMID: 38366641
- PMCID: PMC11142340
- DOI: 10.1093/plphys/kiae088
Far-red light-enhanced apical dominance stimulates flower and fruit abortion in sweet pepper
Abstract
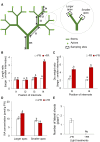
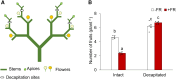
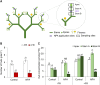
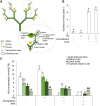
Far-red radiation affects many plant processes, including reproductive organ abortion. Our research aimed to determine the role of apical dominance in far-red light-induced flower and fruit abortion in sweet pepper (Capsicum annuum L.). We conducted several climate room experiments where plants were grown under white- or red-rich LED light, with or without additional far-red light. Additional far-red light enhanced apical dominance: it increased auxin levels in the apices of dominant shoots, and caused a greater difference in internode length and apical auxin levels between dominant and subordinate shoots. Additional far-red light stimulated fruit abortion in intact plants but not in decapitated plants, suggesting a crucial role of shoot apices in this effect. However, reducing basipetal auxin transport in the stems with N-1-naphthylphthalamic acid did not influence far-red light-stimulated fruit abortion, although auxin levels in the stem were largely reduced. Applying the synthetic auxin 1-naphthaleneacetic acid on decapitated apices did not influence fruit abortion. However, applying the auxin biosynthesis inhibitor yucasin to shoot apices reduced fruit abortion regardless of the light conditions, accompanied by slight shoot growth retardation. These findings suggest that the basipetal auxin stream does not mediate far-red light-stimulated fruit abortion. Far-red light-stimulated fruit abortion was associated with reduced sucrose accumulation and lower invertase activities in flowers. We suggest that under additional far-red light conditions, increased auxin levels in shoot apices promote fruit abortion probably through enhanced competition for assimilates between apices and flowers, which limits assimilate import into flowers.
© The Author(s) 2024. Published by Oxford University Press on behalf of American Society of Plant Biologists.
Conflict of interest statement
Conflict of interest statement. None declared.
Figures

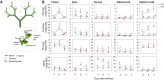
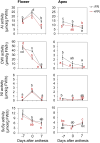
References
-
- Aloni B, Karni L, Zaidman Z, Schaffer AA. Changes of carbohydrates in pepper (Capsicum annuum L.) flowers in relation to their abscission under different shading regimes. Ann Bot. 1996:78(2):163–168. 10.1006/anbo.1996.0109 - DOI
-
- Aloni B, Karni L, Zaidman Z, Schaffer AA. The relationship between sucrose supply, sucrose-cleaving enzymes and flower abortion in pepper. Ann Bot. 1997:79(6):601–605. 10.1006/anbo.1996.0410 - DOI
-
- Aloni B, Pashkar T, Karni L. Partitioning of [14C] sucrose and acid invertase activity in reproductive organs of pepper plants in relation to their abscission under heat stress. Ann Bot. 1991:67(5):371–377. 10.1093/oxfordjournals.aob.a088170 - DOI
-
- Bangerth F. Dominance among fruits/sinks and the search for a correlative signal. Physiol Plant. 1989:76(4):608–614. 10.1111/j.1399-3054.1989.tb05487.x - DOI
-
- Bangerth F. Abscission and thinning of young fruit and their regulation by plant hormones and bioregulators. Plant Growth Regul. 2000:31(1/2):43–59. 10.1023/A:1006398513703 - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

