Heat stress at the bicellular stage inhibits sperm cell development and transport into pollen tubes
- PMID: 38366643
- PMCID: PMC11213256
- DOI: 10.1093/plphys/kiae087
Heat stress at the bicellular stage inhibits sperm cell development and transport into pollen tubes
Abstract
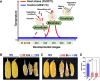
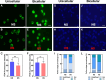
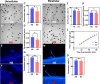
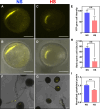
For successful double fertilization in flowering plants (angiosperms), pollen tubes deliver 2 nonmotile sperm cells toward female gametes (egg and central cell, respectively). Heatwaves, especially during the reproduction period, threaten male gametophyte (pollen) development, resulting in severe yield losses. Using maize (Zea mays) as a crop and grass model system, we found strong seed set reduction when moderate heat stress was applied for 2 d during the uni- and bicellular stages of pollen development. We show that heat stress accelerates pollen development and impairs pollen germination capabilities when applied at the unicellular stage. Heat stress at the bicellular stage impairs sperm cell development and transport into pollen tubes. To understand the course of the latter defects, we used marker lines and analyzed the transcriptomes of isolated sperm cells. Heat stress affected the expression of genes associated with transcription, RNA processing and translation, DNA replication, and the cell cycle. This included the genes encoding centromeric histone 3 (CENH3) and α-tubulin. Most genes that were misregulated encode proteins involved in the transition from metaphase to anaphase during pollen mitosis II. Heat stress also activated spindle assembly check point and meta- to anaphase transition genes in sperm cells. In summary, misregulation of the identified genes during heat stress at the bicellular stage results in sperm cell development and transport defects ultimately leading to sterility.
© The Author(s) 2024. Published by Oxford University Press on behalf of American Society of Plant Biologists.
Conflict of interest statement
Conflict of interest statement. None declared.
Figures

References
-
- Ahmadli U, Kalidass M, Khaitova LC, Fuchs J, Cuacos M, Demidov D, Zuo S, Pecinkova J, Mascher M, Ingouff M, et al. . High temperature increases centromere-mediated genome elimination frequency and enhances haploid induction in Arabidopsis. Plant Commun. 2023:4(3):100507. 10.1016/j.xplc.2022.100507 - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

