Green industry work: production of FeCl3 from iron and steel industry waste (mill scale) and its use in wastewater treatment
- PMID: 38367113
- PMCID: PMC10927800
- DOI: 10.1007/s11356-024-32451-6
Green industry work: production of FeCl3 from iron and steel industry waste (mill scale) and its use in wastewater treatment
Abstract
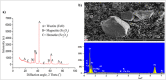
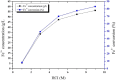
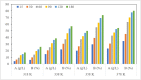
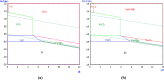
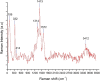
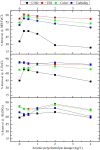
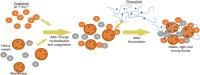
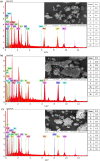
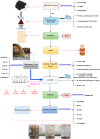
Mill scale (MS) is considered to be a significant metallurgical waste, but there is no economical method yet to utilize its metal content. In this study, which covers various processes in several stages, the solution of iron in MS, which is the Iron and Steel Industry (I&SI) waste, as FeCl3 (MS-FeCl3) in the thermoreactor in the presence of HCl, was investigated. In the next step, the conditions for using this solution as a coagulant in the treatment of I&SI wastewater were investigated using the jar test. The results of the treated water sample were compared by chemical oxygen demand (COD), total suspended solids (TSS), color, and turbidity analyses using commercial aluminum sulfate (Al2(SO4)3) and FeCl3 (C-FeCl3). Additionally, heavy metal analyses were conducted, and the treatment performance of three coagulants was presented. Accordingly, while 2.0 mg/L anionic polyelectrolyte was consumed at a dosage of 4.05 mg/L Al2(SO4)3 at pH 7.0, 0.25 mg/L anionic polyelectrolyte was consumed at a dosage of 1.29 mg/L at pH 5.0 in the C-FeCl3 and MS-FeCl3 studies. Also, Fe, Cr, Mn, Ni, Zn, Cd, Hg, and Pb removal efficiencies were over 93.56% for all three coagulant usage cases. The results showed that the wastewater treatment performance of MS-FeCl3 by the recycling of MS, which is an I&SI waste, was at the same level as C-FeCl3. Thus, thanks to recycling, waste scale can be used as an alternative to commercial products for green production.
Keywords: Coagulation-flocculation; FeCl3 production; Heavy metal; Iron and steel wastewater; Mill scale.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

References
-
- Abujazar MSS, Karaağaç SU, Abu Amr SS, et al. Recent advancement in the application of hybrid coagulants in coagulation-flocculation of wastewater: a review. J Clean Prod. 2022;345:131133. doi: 10.1016/J.JCLEPRO.2022.131133. - DOI
-
- Abujazar MSS, Karaağaç SU, Amr SSA, et al. The effectiveness of rosehip seeds powder as a plant-based natural coagulant for sustainable treatment of steel industries wastewater. Desalination Water Treat. 2022;270:44–51. doi: 10.5004/DWT.2022.28782. - DOI
-
- Akbal F, Camci S. Comparison of electrocoagulation and chemical coagulation for heavy metal removal. Chem Eng Technol. 2010;33:1655–1664. doi: 10.1002/CEAT.201000091. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

