Reconstructed Human Skin with Hypodermis Shows Essential Role of Adipose Tissue in Skin Metabolism
- PMID: 38367122
- PMCID: PMC10987437
- DOI: 10.1007/s13770-023-00621-1
Reconstructed Human Skin with Hypodermis Shows Essential Role of Adipose Tissue in Skin Metabolism
Abstract
Background: Dysregulation of skin metabolism is associated with a plethora of diseases such as psoriasis and dermatitis. Until now, reconstructed human skin (RhS) models lack the metabolic potential of native human skin, thereby limiting their relevance to study human healthy and diseased skin. We aimed to determine whether incorporation of an adipocyte-containing hypodermis into RhS improves its metabolic potential and to identify major metabolic pathways up-regulated in adipose-RhS.
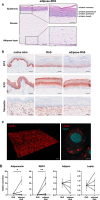
Methods: Primary human keratinocytes, fibroblasts and differentiated adipose-derived stromal cells were co-cultured in a collagen/fibrin scaffold to create an adipose-RhS. The model was extensively characterized structurally in two- and three-dimensions, by cytokine secretion and RNA-sequencing for metabolic enzyme expression.
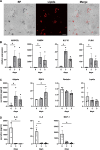
Results: Adipose-RhS showed increased secretion of adipokines. Both RhS and adipose-RhS expressed 29 of 35 metabolic genes expressed in ex vivo native human skin. Addition of the adipose layer resulted in up-regulation of 286 genes in the dermal-adipose fraction of which 7 were involved in phase I (CYP19A1, CYP4F22, CYP3A5, ALDH3B2, EPHX3) and phase II (SULT2B1, GPX3) metabolism. Vitamin A, D and carotenoid metabolic pathways were enriched. Additionally, pro-inflammatory (IL-1β, IL-18, IL-23, IL-33, IFN-α2, TNF-α) and anti-inflammatory cytokine (IL-10, IL-12p70) secretion was reduced in adipose-RhS.
Conclusions: Adipose-RhS mimics healthy native human skin more closely than traditional RhS since it has a less inflamed phenotype and a higher metabolic activity, indicating the contribution of adipocytes to tissue homeostasis. Therefore it is better suited to study onset of skin diseases and the effect of xenobiotics.
Keywords: Adipocytes; Metabolism; Organotypic models; Skin substitutes; Subcutaneous adipose tissue.
© 2024. The Author(s).
Conflict of interest statement
RKB is employed by Unilever R&D Colworth. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Systemic Dermatitis Model Mice Exhibit Atrophy of Visceral Adipose Tissue and Increase Stromal Cells via Skin-Derived Inflammatory Cytokines.Int J Mol Sci. 2020 May 9;21(9):3367. doi: 10.3390/ijms21093367. Int J Mol Sci. 2020. PMID: 32397568 Free PMC article.
-
Pro-inflammatory cytokines and adipose tissue.Proc Nutr Soc. 2001 Aug;60(3):349-56. doi: 10.1079/pns2001110. Proc Nutr Soc. 2001. PMID: 11681809 Review.
-
Engineering a Multilayered Skin Substitute with Keratinocytes, Fibroblasts, Adipose-Derived Stem Cells, and Adipocytes.Methods Mol Biol. 2019;1993:149-157. doi: 10.1007/978-1-4939-9473-1_12. Methods Mol Biol. 2019. PMID: 31148085
-
Bio-engineering a prevascularized human tri-layered skin substitute containing a hypodermis.Acta Biomater. 2021 Oct 15;134:215-227. doi: 10.1016/j.actbio.2021.07.033. Epub 2021 Jul 21. Acta Biomater. 2021. PMID: 34303011
-
Release of interleukins and other inflammatory cytokines by human adipose tissue is enhanced in obesity and primarily due to the nonfat cells.Vitam Horm. 2006;74:443-77. doi: 10.1016/S0083-6729(06)74018-3. Vitam Horm. 2006. PMID: 17027526 Review.
Cited by
-
A bioactive three-layered skin substitute based on ECM components effectively promotes skin wound healing and regeneration.Mater Today Bio. 2025 Feb 20;31:101592. doi: 10.1016/j.mtbio.2025.101592. eCollection 2025 Apr. Mater Today Bio. 2025. PMID: 40092225 Free PMC article.
-
Transethosomes: A Comprehensive Review of Ultra-Deformable Vesicular Systems for Enhanced Transdermal Drug Delivery.AAPS PharmSciTech. 2025 Jan 17;26(1):41. doi: 10.1208/s12249-024-03035-x. AAPS PharmSciTech. 2025. PMID: 39825015 Review.
-
Advancements in bioengineered and autologous skin grafting techniques for skin reconstruction: a comprehensive review.Front Bioeng Biotechnol. 2025 Jan 7;12:1461328. doi: 10.3389/fbioe.2024.1461328. eCollection 2024. Front Bioeng Biotechnol. 2025. PMID: 39840132 Free PMC article.
-
In vitro immunity: an overview of immunocompetent organ-on-chip models.Front Immunol. 2024 May 21;15:1373186. doi: 10.3389/fimmu.2024.1373186. eCollection 2024. Front Immunol. 2024. PMID: 38835750 Free PMC article. Review.
-
Environmentally Controlled Microfluidic System Enabling Immune Cell Flow and Activation in an Endothelialised Skin-On-Chip.Adv Healthc Mater. 2024 Nov;13(29):e2400750. doi: 10.1002/adhm.202400750. Epub 2024 Oct 6. Adv Healthc Mater. 2024. PMID: 39370595 Free PMC article.
References
-
- Mukhtar H, Bickers DR. Drug metabolism in skin. Comparative activity of the mixed-function oxidases, epoxide hydratase, and glutathione S-transferase in liver and skin of the neonatal rat. Drug Metab Dispos. 1981;9:311–4. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
