Comparison of trastuzumab emtansine, trastuzumab deruxtecan, and disitamab vedotin in a multiresistant HER2-positive breast cancer lung metastasis model
- PMID: 38367127
- PMCID: PMC10973002
- DOI: 10.1007/s10585-024-10278-2
Comparison of trastuzumab emtansine, trastuzumab deruxtecan, and disitamab vedotin in a multiresistant HER2-positive breast cancer lung metastasis model
Abstract
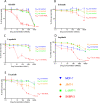
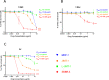
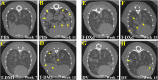
Human epidermal growth factor 2 (HER2)-positive breast cancer with lung metastases resistant to targeted agents is a common therapeutic challenge. Absence of preclinical lung metastasis models that are resistant to multiple anti-HER2 targeted drugs hampers the development of novel therapies. We established a novel HER2-positive breast cancer cell line (L-JIMT-1) with a high propensity to form lung metastases from the parenteral JIMT-1 cell line by injecting JIMT-1 cells into immunodeficient SCID mice. Lung metastases developed in all mice injected with L-JIMT-1 cells, and more rapidly and in greater numbers compared with the parental JIMT-1 cells. L-JIMT-1 cells expressed more epidermal growth factor receptor and HER2 than JIMT-1 cells. L-JIMT-1 cells were resistant to all five tyrosine kinase inhibitors tested in vitro (afatinib, erlotinib, lapatinib, sapitinib, and tucatinib). When we compared JIMT-1 and L-JIMT-1 sensitivity to three HER2-targeting antibody-drug conjugates (ADCs) trastuzumab emtansine (T-DM1), trastuzumab deruxtecan (T-DXd), and disitamab vedotin (DV) in vitro, JIMT-1 cells were resistant T-DXd, partially sensitive to T-DM1, and sensitive to DV, while L-JIMT-1 cells were resistant to both T-DM1 and T-DXd, but moderately sensitive to DV. In a mouse model, all three ADCs inhibited the growth of L-JIMT-1 lung metastases compared to a vehicle, but DV and T-DXd more strongly than T-DM1, and DV treatment led to the smallest tumor burden. The L-JIMT breast cancer lung metastasis model developed may be useful in the evaluation of anti-cancer agents for multiresistant HER2-positive advanced breast cancer.
Keywords: Antibody drug conjugate; Breast cancer; Disitamab vedotin; Human epidermal growth factor receptor 2; Lung metastasis; Trastuzumab deruxtecan; Trastuzumab emtansine.
© 2024. The Author(s).
Conflict of interest statement
H. Joensuu has an advisory board relationship at Orion Pharma, Neutron Therapeutics, and Maud Kuistila Foundation, has ownership interest (including patents) at Sartar Therapeutics, has received a honorarium for presentation from Deciphera Pharmaceuticals. M. Barok and H. Joensuu report grants from Mersana Therapeutics, Defense Therapeutics, and Merck KGaA outside the submitted work. No potential conflicts of interest were disclosed by the other authors.
Figures



References
-
- Hurvitz SA, Dirix L, Kocsis J, Bianchi GV, Lu J, Vinholes J, et al. Phase II randomized study of trastuzumab emtansine versus trastuzumab plus docetaxel in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer. J Clin Oncol. 2013;31(9):1157–1163. doi: 10.1200/JCO.2012.44.9694. - DOI - PubMed
-
- Hurvitz SA, Hegg R, Chung WP, Im SA, Jacot W, Ganju V, et al. Trastuzumab deruxtecan versus trastuzumab emtansine in patients with HER2-positive metastatic breast cancer: updated results from DESTINY-Breast03, a randomised, open-label, phase 3 trial. Lancet. 2023;401(10371):105–117. doi: 10.1016/S0140-6736(22)02420-5. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

