Pharmacokinetic-Pharmacodynamic Modelling in Hemophilia A: Relating Thrombin and Plasmin Generation to Factor VIII Activity After Administration of a VWF/FVIII Concentrate
- PMID: 38367175
- PMCID: PMC10904421
- DOI: 10.1007/s13318-024-00876-6
Pharmacokinetic-Pharmacodynamic Modelling in Hemophilia A: Relating Thrombin and Plasmin Generation to Factor VIII Activity After Administration of a VWF/FVIII Concentrate
Abstract
Background: Hemophilia A patients are treated with factor (F) VIII prophylactically to prevent bleeding. In general, dosage and frequency are based on pharmacokinetic measurements. Ideally, an alternative dose adjustment can be based on the hemostatic potential, measured with a thrombin generation assay (TGA), like the Nijmegen hemostasis assay.
Objective: The objective of this study was to investigate the predicted performance of a previously developed pharmacokinetic-pharmacodynamic model for FVIII replacement therapy, relating FVIII dose and FVIII activity levels with thrombin and plasmin generation parameters.
Methods: Pharmacokinetic and pharmacodynamic measurements were obtained from 29 severe hemophilia A patients treated with pdVWF/FVIII concentrate (Haemate P®). The predictive performance of the previously developed pharmacokinetic-pharmacodynamic model was evaluated using nonlinear mixed-effects modeling (NONMEM). When predictions of FVIII activity or TGA parameters were inadequate [median prediction error (MPE) > 20%], a new model was developed.
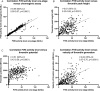
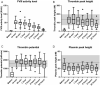
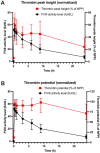
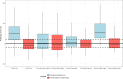
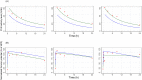
Results: The original pharmacokinetic model underestimated clearance and was refined based on a two-compartment model. The pharmacodynamic model displays no bias in the observed normalized thrombin peak height and normalized thrombin potential (MPE of 6.83% and 7.46%). After re-estimating pharmacodynamic parameters, EC50 and Emax values were relatively comparable between the original model and this group. Prediction of normalized plasmin peak height was inaccurate (MPE 58.9%).
Conclusion: Our predictive performance displayed adequate thrombin pharmacodynamic predictions of the original model, but a new pharmacokinetic model was required. The pharmacodynamic model is not factor specific and applicable to multiple factor concentrates. A prospective study is needed to validate the impact of the FVIII dosing pharmacodynamic model on bleeding reduction in patients.
© 2024. The Author(s).
Conflict of interest statement
MC was funded by a grant from the Netherlands Organisation for Scientific Research (NWO) in the framework of the NWA-ORC Call grant agreement NWA.1160.18.038. MHC has received grants outside the submitted work from governmental research institutes such as NWO: ZonMW and NWO-NWA and the Innovation Fund, and an unrestricted investigator initiated research grants as well as educational and travel funding from the following companies over the years: Pfizer, Baxter/Baxalta/Shire, Bayer Schering Pharma, CSL Behring, Sobi Biogen, Novo Nordisk, Novartis, and Nordic Pharma, and has served as a member on steering boards of Roche, Bayer, and Octapharma. All grants, awards, and fees are always collected by the institution. RM has received grants from governmental and societal research institutes such as NWO, ZonMW, and Innovation fund and unrestricted investigator research grants from Baxter, Baxalta, Shire, Takeda, Bayer, CSL Behring, and Sobi. He has served as advisor for Bayer, CSL Behring, Merck Sharp & Dohme, Baxter, Baxalta, Shire, and Takeda. All grants and fees were paid to the institution. WvH received unrestricted grants from Bayer, Takeda, Novo Nordisk, and CSL Behring. WvH is the co-founder and CSO of Enzyre BV, a Radboudumc spinoff company. LV, MC, WB, HM, NB, and SS have no conflicts of interest to declare.
Figures
Similar articles
-
Combining factor VIII levels and thrombin/plasmin generation: A population pharmacokinetic-pharmacodynamic model for patients with haemophilia A.Br J Clin Pharmacol. 2022 Jun;88(6):2757-2768. doi: 10.1111/bcp.15185. Epub 2022 Jan 15. Br J Clin Pharmacol. 2022. PMID: 34921439 Free PMC article.
-
Pharmacodynamic monitoring of factor VIII replacement therapy in hemophilia A: Combining thrombin and plasmin generation.J Thromb Haemost. 2020 Dec;18(12):3222-3231. doi: 10.1111/jth.15106. Epub 2020 Oct 21. J Thromb Haemost. 2020. PMID: 32979031 Free PMC article.
-
VWF/FVIII complex and the management of patient with inhibitors: from laboratory to clinical practice.Haemophilia. 2007 Dec;13 Suppl 5:69-72. doi: 10.1111/j.1365-2516.2007.01577.x. Haemophilia. 2007. PMID: 18078401
-
Management of von Willebrand disease with factor VIII/von Willebrand factor concentrates: results from current studies and surveys.Blood Coagul Fibrinolysis. 2005 Apr;16 Suppl 1:S17-21. doi: 10.1097/01.mbc.0000167658.85143.49. Blood Coagul Fibrinolysis. 2005. PMID: 15849522 Review.
-
Efanesoctocog alfa for the prevention and treatment of bleeding in patients with hemophilia A.Expert Rev Hematol. 2023 Jul-Dec;16(8):567-573. doi: 10.1080/17474086.2023.2223925. Epub 2023 Jun 15. Expert Rev Hematol. 2023. PMID: 37289594 Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

