Harnessing the Potential of Real-World Evidence in the Treatment of Colorectal Cancer: Where Do We Stand?
- PMID: 38367182
- PMCID: PMC10997699
- DOI: 10.1007/s11864-024-01186-4
Harnessing the Potential of Real-World Evidence in the Treatment of Colorectal Cancer: Where Do We Stand?
Abstract
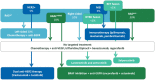
Treatment guidelines for colorectal cancer (CRC) are primarily based on the results of randomized clinical trials (RCTs), the gold standard methodology to evaluate safety and efficacy of oncological treatments. However, generalizability of trial results is often limited due to stringent eligibility criteria, underrepresentation of specific populations, and more heterogeneity in clinical practice. This may result in an efficacy-effectiveness gap and uncertainty regarding meaningful benefit versus treatment harm. Meanwhile, conduct of traditional RCTs has become increasingly challenging due to identification of a growing number of (small) molecular subtypes. These challenges-combined with the digitalization of health records-have led to growing interest in use of real-world data (RWD) to complement evidence from RCTs. RWD is used to evaluate epidemiological trends, quality of care, treatment effectiveness, long-term (rare) safety, and quality of life (QoL) measures. In addition, RWD is increasingly considered in decision-making by clinicians, regulators, and payers. In this narrative review, we elaborate on these applications in CRC, and provide illustrative examples. As long as the quality of RWD is safeguarded, ongoing developments, such as common data models, federated learning, and predictive modelling, will further unfold its potential. First, whenever possible, we recommend conducting pragmatic trials, such as registry-based RCTs, to optimize generalizability and answer clinical questions that are not addressed in registrational trials. Second, we argue that marketing approval should be conditional for patients who would have been ineligible for the registrational trial, awaiting planned (non) randomized evaluation of outcomes in the real world. Third, high-quality effectiveness results should be incorporated in treatment guidelines to aid in patient counseling. We believe that a coordinated effort from all stakeholders is essential to improve the quality of RWD, create a learning healthcare system with optimal use of trials and real-world evidence (RWE), and ultimately ensure personalized care for every CRC patient.
Keywords: Colorectal cancer; Efficacy-effectiveness gap; Oncology; Population-based; Real-world data; Real-world evidence.
© 2024. The Author(s).
Conflict of interest statement
S.N. reports receiving support for travel from Servier via institution outside the submitted work. G.B. reports a patent for biomarker DDX3, receiving institutional grants from Bayer, Terumo and Servier, and honoraria for lectures from Pierre Fabre, Terumo and Bayer outside the submitted work. F.B. reports receiving an institutional grant from Personal Genome Diagnostics (PGDx) outside the submitted work. J.R. reports receiving institutional grants from Bristol Myers Squibb, Pierre Fabre, Servier, HUB 4 organoids, Clear Biotech, GSK, Cilis biotech, DoMore diagnostics, receiving consulting fees via institution from Bayer, Bristol Myers Squibb, Merck-Serono, Pierre Fabre, Servier, GSK, Amgen, has received payment via institution for lectures from Bristol Myers Squibb, Pierre Fabre and Servier, support for meetings and/or travel from Servier, participates on a Data Safety Monitoring Board or Advisory Board for PELVEX and MENDIT, and non-remunerated activity in the ONCODE clinical advisory board, KWF scientific board, and Hubrecht Organoid Biobank foundation management board, all outside the submitted work. G.V. reports receiving institutional grants from PGDx, Delfi diagnostics, Merck, Servier, Bayer, Bristol Myers Squibb, Sirtex and Pierre Fabre outside the submitted work. C.P. reports receiving consulting fees via institution from Nordic Pharma, outside the submitted work.
M.K. reports institutional scientific grants from Bayer, Bristol Myers Squibb, Merck, PGDx, Pierre Fabre, Roche, Sirtex and Servier, has an advisory role for Eisai, Nordic Farma, Merck-Serono, Pierre Fabre, and Servier, is principal investigator of the Prospective Dutch CRC (PLCRC) cohort and the international cohort study PROMETCO with Servier as sponsor, and reports non-remunerated activity as vice-chair for the Dutch Colorectal Cancer Group, chair of the ESMO Real World Data and Digital Health Working Group, and involvement in several colorectal cancer clinical trials as PI or co-investigator, all outside the submitted work. A.M. and J.D. declare no conflict of interest.
Figures
References
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
-
- Ayaz A, Naqvi SAA, Islam M, Ikram W, Raza U, Riaz A, et al. Source of funding and enrollment disparities in the inclusion of minorities in colorectal clinical trials: a systematic review and meta-analysis. Journal of Clinical Oncology. 2023;41(4_suppl):23–23. doi: 10.1200/JCO.2023.41.4_suppl.23. - DOI
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous