High Target Homology Does Not Guarantee Inhibition: Aminothiazoles Emerge as Inhibitors of Plasmodium falciparum
- PMID: 38367280
- PMCID: PMC10928712
- DOI: 10.1021/acsinfecdis.3c00670
High Target Homology Does Not Guarantee Inhibition: Aminothiazoles Emerge as Inhibitors of Plasmodium falciparum
Abstract
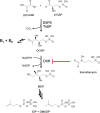
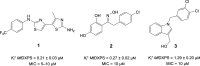
In this study, we identified three novel compound classes with potent activity against Plasmodium falciparum, the most dangerous human malarial parasite. Resistance of this pathogen to known drugs is increasing, and compounds with different modes of action are urgently needed. One promising drug target is the enzyme 1-deoxy-d-xylulose-5-phosphate synthase (DXPS) of the methylerythritol 4-phosphate (MEP) pathway for which we have previously identified three active compound classes against Mycobacterium tuberculosis. The close structural similarities of the active sites of the DXPS enzymes of P. falciparum and M. tuberculosis prompted investigation of their antiparasitic action, all classes display good cell-based activity. Through structure-activity relationship studies, we increased their antimalarial potency and two classes also show good metabolic stability and low toxicity against human liver cells. The most active compound 1 inhibits the growth of blood-stage P. falciparum with an IC50 of 600 nM. The results from three different methods for target validation of compound 1 suggest no engagement of DXPS. All inhibitor classes are active against chloroquine-resistant strains, confirming a new mode of action that has to be further investigated.
Keywords: DXPS; MEP pathway; Plasmodium falciparum; Polypharmacology Browser; malaria.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

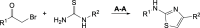

References
-
- World Health Organization . World Malaria Report 2021, 2021.
-
- World Health Organization . Status report on artemisinin resistance and ACT, World Health Organization: Geneva: 2018.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

