Does size matter? - Comparing pyranoses with septanoses as ligands of the bacterial lectin FimH
- PMID: 38367495
- PMCID: PMC10964925
- DOI: 10.1016/j.ejmech.2024.116225
Does size matter? - Comparing pyranoses with septanoses as ligands of the bacterial lectin FimH
Abstract
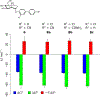
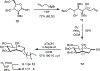
The pharmacological modulation of disease-relevant carbohydrate-protein interactions represents an underexplored area of medicinal chemistry. One particular challenge in the design of glycomimetic compounds is the inherent instability of the glycosidic bond toward enzymatic cleavage. This problem has traditionally been approached by employing S-, N-, or C-glycosides with reduced susceptibility toward glycosidases. The application of ring-extended glycomimetics is an innovative approach to circumvent this issue. On the example of the bacterial adhesin FimH, it was explored how design principles from pyranose glycomimetics transfer to analogous septanose structures. A series of ring-extended FimH antagonists exhibiting the well-proven pharmacophore necessary for targeting the tyrosine-gate of FimH was synthesized. The resulting septanoses were evaluated for their affinity to the conformationally rigid isolated lectin domain of FimH (FimHLD), as well as a structurally flexible full-length FimH (FimHFL) construct. Some elements of potent mannoside-based FimH antagonists could be successfully transferred to septanose-based ligands, ultimately resulting in a 32-fold increase in binding affinity. Interestingly, the canonical ca. 100-fold loss of binding affinity between FimHLD and FimHFL is partly mitigated by the more flexible septanose antagonists, hinting at potentially differing interaction features of the flexible glycomimetics with intermediately populated states during the conformational transition of FimHFL.
Keywords: Antiadhesive therapy; Bacterial lectin; Glycomimetics; Septanose; Urinary tract infections.
Copyright © 2024 Elsevier Masson SAS. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
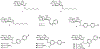





References
-
- Werle M, Bernkop-Schnurch A, Amino Acids 2006, 30, 351–367. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

