Under-representation of the WHO African region in clinical trials of interventions against hepatitis B virus infection
- PMID: 38367632
- PMCID: PMC7616036
- DOI: 10.1016/S2468-1253(23)00315-1
Under-representation of the WHO African region in clinical trials of interventions against hepatitis B virus infection
Abstract
The WHO African region bears a disproportionate burden of morbidity and mortality related to chronic hepatitis B virus (HBV) infection and accounts for an estimated 70% of new HBV infections worldwide. We investigated the extent to which HBV clinical trials represented populations in this region by searching the WHO International Clinical Trials Registry Platform and ClinicalTrials.gov for interventional clinical trials published in English between database inception and May 29, 2023, using the search term "Hepatitis B". We identified 1804 unique clinical trials, of which 18 (1·0%) recorded involvement of the WHO African region. There is no evidence that the number of HBV clinical trials in this region has improved over time. The diversity of new interventions and industry sponsorship in the WHO African region were low, with trials of HBV comparing poorly with those of other endemic infectious diseases (eg, malaria, HIV, and SARS-CoV-2). HBV research and clinical trial investigations have neglected the WHO African region, leading to profound health inequities. HBV clinical trials are urgently needed to evaluate the efficacy of newly discovered therapeutics and to ensure that interventions can be equitably distributed and deployed as they become available.
Copyright © 2024 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of interests MD, EW, and PCM receive funding from The Francis Crick Institute. PCM is funded by a Wellcome fellowship (ref 110110/Z/15/Z) and the University College London National Institute for Health and Care Research Biomedical Research Centre and receives funding from GlaxoSmithKline to support a doctoral student in her team. LOD and SFL receive doctoral funding from the Wellcome Trust. CI has received research grant funding from Gilead Sciences. All other authors declare no competing interests. Editorial note: The Lancet Group takes a neutral position with respect to territorial claims in published maps, text, and institutional affiliations.
Figures
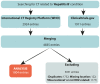
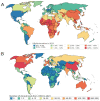



Comment in
-
Equity in clinical trials for hepatitis B.Lancet Gastroenterol Hepatol. 2024 Jun;9(6):501-502. doi: 10.1016/S2468-1253(24)00083-9. Lancet Gastroenterol Hepatol. 2024. PMID: 38734004 No abstract available.
References
-
- Hepatitis B vaccines: WHO position paper – July 2017. [Internet]. [08.30.2023] Available from : https://www.who.int/publications-detail-redirect/WER9227.
-
- Overview | Hepatitis B (chronic): diagnosis and management | Guidance | NICE. NICE; 2013. [Internet] [08.30.2023]. Available from: https://www.nice.org.uk/guidance/cg165.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

