Development and evaluation of a simple treatment eligibility score (HEPSANET) to decentralise hepatitis B care in Africa: a cross-sectional study
- PMID: 38367633
- PMCID: PMC7616035
- DOI: 10.1016/S2468-1253(23)00449-1
Development and evaluation of a simple treatment eligibility score (HEPSANET) to decentralise hepatitis B care in Africa: a cross-sectional study
Abstract
Background: Hepatitis B virus (HBV) elimination requires expanding and decentralising HBV care services. However, peripheral health facilities lack access to diagnostic tools to assess eligibility for antiviral therapy. Through the Hepatitis B in Africa Collaborative Network (HEPSANET), we aimed to develop and evaluate a score using tests generally available at lower-level facilities, to simplify the evaluation of antiviral therapy eligibility in people living with HBV.
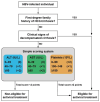
Methods: We surveyed the availability of clinical and laboratory parameters across different health-care levels in sub-Saharan Africa. We used data from the HEPSANET dataset, the largest cross-sectional dataset of treatment-naive people living with HBV in sub-Saharan Africa, to derive and validate the score. Participants from this dataset were included in the analysis if they were aged 18 years or older and had liver fibrosis stages determined by a liver stiffness measurement or liver histopathology. Participants with co-infections or metabolic disorders were excluded. We allocated participants to the derivation and validation sets by geographical site. In the derivation set, we used stepwise logistic regression to identify the best performing parameters for identifying participants that met the 2017 European Association for the Study of the Liver (EASL) criteria. Regression coefficients were converted into integer points to construct simplified algorithms for different health-care levels. In the validation set, we estimated the area under the receiver operating characteristic, sensitivity, and specificity of the simplified algorithm for identifying antiviral therapy eligibility defined by the 2017 EASL criteria.
Findings: At 11 sites from eight countries that returned surveys, aspartate aminotransferase (AST), alanine aminotransferase (ALT), and platelet count were generally available at district hospital levels, and hepatitis B e antigen and point-of-care HBV DNA tests were available only at regional and provincial hospital levels or above. Among 2895 participants included from the HEPSANET database (1740 [60·1%] male, 1155 [39·9%] female), 409 (14·1%) met EASL antiviral therapy eligibility criteria. In the derivation set, the optimal district-level hospital score was: ALT (IU/L), less than 40 (0 points), 40-79 (+1), 80 or greater (+2); AST (IU/L), less than 40 (0), 40-79 (+1), 80 or greater (+2); and platelet counts (109/L), less than 100 (+2), 100-149 (+1), 150 or greater (0). When combined with family history and clinical data for decompensated cirrhosis that do not require any biological tests, a cut-off of 2 points or more had a sensitivity and specificity of 82% (95% CI 76-86) and 95% (93-96) to identify treatment-eligible individuals in the derivation set, and 78% (71-85) and 87% (86-89) in the validation set, respectively.
Interpretation: Using a score incorporating platelet counts, AST, and ALT, the majority of people living with HBV requiring antiviral therapy can be identified. Our findings suggest that clinical staging can be decentralised down to district hospital level in sub-Saharan Africa.
Funding: European Association for the Study of the Liver Foundation, John C Martin Foundation.
Translation: For the French translation of the abstract see Supplementary Materials section.
Copyright © 2024 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of interests CWS has received speaker bureau honoraria from Gilead Sciences and Abbott, as well as travel support from Gilead Sciences. ES has received a research grant from Gilead Sciences. GW has received a research grant from Roche Diagnostics, Roche Molecular, and Gilead Sciences, and consulting fees from Gilead Sciences, Merck Sharp and Dohme, and ViiV. MA has received research grants from Prenetics, Pfizer, and Johnson & Johnson; consulting fees from Prenetics; honoraria from Pfizer; travel support from WHO; reports planned patents for Ramanomics and LAMP GC/CT/HSV/HBV; and is an unpaid board member of the Charity E3 initiative. MSo has received speaker bureau honoraria from Roche and Gilead Sciences, and travel support from Gilead Sciences. MV has received funding from the US National Institutes of Health (R01AI147727), and travel support from Africa Centres for Disease Control and Prevention. PCM has received funding from the Wellcome Trust (110110) and the Francis Crick Institute, and also reports sponsorship from GSK for a doctoral student, outside the scope of the current work. YS has received a research grant and honoraria for lectures from Gilead Sciences and research materials from Abbott Laboratories and Fujirebio. All other authors declare no competing interests.
Figures
Comment in
-
A new tool for assessing hepatitis B treatment eligibility in Africa.Lancet Gastroenterol Hepatol. 2024 Apr;9(4):277-278. doi: 10.1016/S2468-1253(24)00006-2. Epub 2024 Feb 15. Lancet Gastroenterol Hepatol. 2024. PMID: 38367630 No abstract available.
-
The HEPSANET score to assess treatment eligibility of chronic hepatitis B in Africa.Lancet Gastroenterol Hepatol. 2024 Jul;9(7):589. doi: 10.1016/S2468-1253(24)00114-6. Lancet Gastroenterol Hepatol. 2024. PMID: 38870962 No abstract available.
References
-
- Razavi-Shearer D, et al. Global prevalence, cascade of care, and prophylaxis coverage of hepatitis B in 2022: a modelling study. Lancet Gastroenterol Hepatol. 2023;8:879–907. - PubMed
-
- McMahon BJ. The natural history of chronic hepatitis B virus infection. Hepatology. 2009;49:S45–S55. - PubMed
-
- WHO. Global progress report on HIV, viral sexually transmitted infections, 2021. World Heal Organ; 2021.
-
- World Health Organization (WHO) Updated recommendations on treatment of adolescents and children with chronic HCV infection, and HCV simplified service delivery and diagnostics. World Health Organization; 2022. pp. 23–25. https://www.who.int/news/item/24-06-2022-WHO-publishes-updated-guidance-... . - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous