Neurophysiological treatment effects of mesdopetam, pimavanserin and clozapine in a rodent model of Parkinson's disease psychosis
- PMID: 38368170
- PMCID: PMC10937958
- DOI: 10.1016/j.neurot.2024.e00334
Neurophysiological treatment effects of mesdopetam, pimavanserin and clozapine in a rodent model of Parkinson's disease psychosis
Abstract
Psychosis in Parkinson's disease is a common phenomenon associated with poor outcomes. To clarify the pathophysiology of this condition and the mechanisms of antipsychotic treatments, we have here characterized the neurophysiological brain states induced by clozapine, pimavanserin, and the novel prospective antipsychotic mesdopetam in a rodent model of Parkinson's disease psychosis, based on chronic dopaminergic denervation by 6-OHDA lesions, levodopa priming, and the acute administration of an NMDA antagonist. Parallel recordings of local field potentials from eleven cortical and sub-cortical regions revealed shared neurophysiological treatment effects for the three compounds, despite their different pharmacological profiles, involving reversal of features associated with the psychotomimetic state, such as a reduction of aberrant high-frequency oscillations in prefrontal structures together with a decrease of abnormal synchronization between different brain regions. Other drug-induced neurophysiological features were more specific to each treatment, affecting network oscillation frequencies and entropy, pointing to discrete differences in mechanisms of action. These findings indicate that neurophysiological characterization of brain states is particularly informative when evaluating therapeutic mechanisms in conditions involving symptoms that are difficult to assess in rodents such as psychosis, and that mesdopetam should be further explored as a potential novel antipsychotic treatment option for Parkinson psychosis.
Keywords: Antipsychotics; Behavior; High-frequency oscillations; In vivo; Local field-potentials.
Copyright © 2024 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Stephan Hjorth, Peder Svensson, Susanna Waters are employed by IRL AB and hold stock in IRL AB. Remaining authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
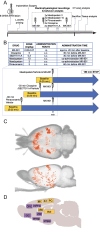
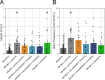
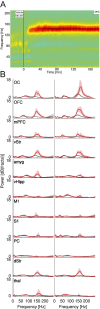
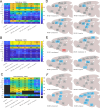

Similar articles
-
Neurophysiological Treatment Effects of Mesdopetam, Pimavanserin and Amantadine in a Rodent Model of Levodopa-Induced Dyskinesia.Eur J Neurosci. 2025 Mar;61(5):e70032. doi: 10.1111/ejn.70032. Eur J Neurosci. 2025. PMID: 40042199 Free PMC article.
-
Evaluating pimavanserin tartrate as a treatment in Parkinson's disease.Expert Opin Pharmacother. 2024 Oct;25(15):1999-2003. doi: 10.1080/14656566.2024.2417733. Epub 2024 Oct 16. Expert Opin Pharmacother. 2024. PMID: 39403823 Review.
-
Behavioral effects of clozapine, pimavanserin, and quetiapine in rodent models of Parkinson's disease and Parkinson's disease psychosis: evaluation of therapeutic ratios.Behav Pharmacol. 2013 Oct;24(7):628-32. doi: 10.1097/FBP.0b013e3283656db6. Behav Pharmacol. 2013. PMID: 23969614
-
[Pimavanserin: a new treatment for the Parkinson's disease psychosis].Tijdschr Psychiatr. 2017;59(9):528-536. Tijdschr Psychiatr. 2017. PMID: 28880354 Review. Dutch.
-
Pharmacological interventions for psychosis in Parkinson's disease patients.Expert Opin Pharmacother. 2018 Apr;19(5):499-505. doi: 10.1080/14656566.2018.1445721. Epub 2018 Mar 1. Expert Opin Pharmacother. 2018. PMID: 29494265 Review.
Cited by
-
Mesdopetam for the Treatment of Levodopa Induced Dyskinesia in Parkinson's Disease: A Randomized Phase 2b Trial.Mov Disord Clin Pract. 2025 Jun;12(6):796-806. doi: 10.1002/mdc3.70004. Epub 2025 Feb 26. Mov Disord Clin Pract. 2025. PMID: 40008823 Free PMC article. Clinical Trial.
-
Global research trends and hotspots in Parkinson's disease psychosis: a 25-year bibliometric and visual analysis.Front Aging Neurosci. 2024 Nov 22;16:1480234. doi: 10.3389/fnagi.2024.1480234. eCollection 2024. Front Aging Neurosci. 2024. PMID: 39649718 Free PMC article.
-
Neurophysiological Treatment Effects of Mesdopetam, Pimavanserin and Amantadine in a Rodent Model of Levodopa-Induced Dyskinesia.Eur J Neurosci. 2025 Mar;61(5):e70032. doi: 10.1111/ejn.70032. Eur J Neurosci. 2025. PMID: 40042199 Free PMC article.
References
-
- Fénelon G., Alves G. Epidemiology of psychosis in Parkinson's disease. J Neurol Sci. 2010 Feb 15;289(1–2):12–17. - PubMed
-
- Forsaa E.B., Larsen J.P., Wentzel-Larsen T., Goetz C.G., Stebbins G.T., Aarsland D., et al. A 12-year population-based study of psychosis in Parkinson disease. Arch Neurol [Internet] 2010 Aug;67(8):996–1001. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

