Challenges and recent advancements in the synthesis of α,α-disubstituted α-amino acids
- PMID: 38368416
- PMCID: PMC10874380
- DOI: 10.1038/s41467-024-45790-2
Challenges and recent advancements in the synthesis of α,α-disubstituted α-amino acids
Abstract
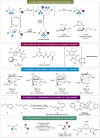
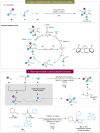
α,α-Disubstituted α-amino acids (α-AAs) have improved properties compared to other types of amino acids. They serve as modifiers of peptide conformation and as precursors of bioactive compounds. Therefore, it has been a long-standing goal to construct this highly valuable scaffold efficiently in organic synthesis and drug discovery. However, access to α,α-disubstituted α-AAs is highly challenging and largely unexplored due to their steric constraints. To overcome these, remarkable advances have been made in the last decades. Emerging strategies such as synergistic enantioselective catalysis, visible-light-mediated photocatalysis, metal-free methodologies and CO2 fixation offer new avenues to access the challenging synthesis of α,α-disubstituted α-AAs and continuously bring additional contributions to this field. This review article aims to provide an overview of the recent advancements since 2015 and discuss existing challenges for the synthesis of α,α-disubstituted α-AAs and their derivatives.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures








References
-
- Koda Y, et al. Similarities of the biological activities of coronatine and coronafacic acid to those of jasmonic acid. Phytochemistry. 1996;41:93–96. doi: 10.1016/0031-9422(95)00517-X. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

