Elucidation of the role of metals in the adsorption and photodegradation of herbicides by metal-organic frameworks
- PMID: 38368421
- PMCID: PMC10874385
- DOI: 10.1038/s41467-024-45546-y
Elucidation of the role of metals in the adsorption and photodegradation of herbicides by metal-organic frameworks
Abstract
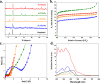
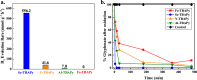
Here, four MOFs, namely Sc-TBAPy, Al-TBAPy, Y-TBAPy, and Fe-TBAPy (TBAPy: 1,3,6,8-tetrakis(p-benzoic acid)pyrene), were characterized and evaluated for their ability to remediate glyphosate (GP) from water. Among these materials, Sc-TBAPy demonstrates superior performance in both the adsorption and degradation of GP. Upon light irradiation for 5 min, Sc-TBAPy completely degrades 100% of GP in a 1.5 mM aqueous solution. Femtosecond transient absorption spectroscopy reveals that Sc-TBAPy exhibits enhanced charge transfer character compared to the other MOFs, as well as suppressed formation of emissive excimers that could impede photocatalysis. This finding was further supported by hydrogen evolution half-reaction (HER) experiments, which demonstrated Sc-TBAPy's superior catalytic activity for water splitting. In addition to its faster adsorption and more efficient photodegradation of GP, Sc-TBAPy also followed a selective pathway towards the oxidation of GP, avoiding the formation of toxic aminomethylphosphonic acid observed with the other M3+-TBAPy MOFs. To investigate the selectivity observed with Sc-TBAPy, electron spin resonance, depleted oxygen conditions, and solvent exchange with D2O were employed to elucidate the role of different reactive oxygen species on GP photodegradation. The findings indicate that singlet oxygen (1O2) plays a critical role in the selective photodegradation pathway achieved by Sc-TBAPy.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures





References
-
- Valavanidis, A. Glyphosate, the most widely used herbicide. Sci. Rev. www.chem-tox-ecotox.org/ScientificReviews (2018).
-
- Zaranyika MF, Nyandoro MG. Degradation of glyphosate in the aquatic environment: An enzymic kinetic model that takes into account microbial degradation of both free and colloidal (or sediment) particle adsorbed glyphosate. J. Agric. Food Chem. 1993;41:838–842. doi: 10.1021/jf00029a030. - DOI
-
- Duke SO. Glyphosate: environmental fate and impact. Weed Sci. 2020;68:201–207. doi: 10.1017/wsc.2019.28. - DOI
LinkOut - more resources
Full Text Sources

