Clinical Landscape of Central Serous Chorioretinopathy in Germany: Retina.net CSC Registry Report Number 1
- PMID: 38368867
- PMCID: PMC11160426
- DOI: 10.1159/000535930
Clinical Landscape of Central Serous Chorioretinopathy in Germany: Retina.net CSC Registry Report Number 1
Abstract
Introduction: The German Registry of central serous chorioretinopathy (CSC) collects data on CSC patients in a nationwide multicenter approach to analyze epidemiology, risk factors, clinical presentations, as well as diagnosis and treatment patterns.
Methods: In this multicenter cohort study, patients with CSC were enrolled in nine tertiary referral centers in Germany between January 2022 and June 2023. After consenting to the study, demographic data, risk factors, reported symptoms, best-corrected visual acuity (BCVA), funduscopic findings, disease severity, and diagnostic and treatment decisions were recorded and analyzed.
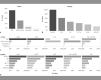
Results: A total of 539 eyes of 411 CSC patients were enrolled in this study including 308 males (75%) and 103 females (25%). Patients were predominantly of Caucasian origin and had a mean age of 55.5 years (IQR 41.0-70.0). 28% of eyes were classified as acute (<4 months duration) CSC, 28% as chronic (>4 months duration) CSC, 21% as inactive CSC, 11% as chronic atrophic CSC, and 12% as CSC with secondary CNV. 128 patients (31%) demonstrated bilateral CSC. The most common risk factors reported were psychological stress (52%), smoking (38%), arterial hypertension (38%), and a history of or current use of steroids (30%). Most frequently encountered symptoms included decreased visual acuity (76%), metamorphopsia (49%), relative scotoma (47%), blurred vision (19%), and dyschromatopsia (9%). The mean logMAR BCVA on initial examination was 0.2 (≈20/30, IQR 0.2-0.4) but showed significant variation with a tendency of lower BCVA in chronic cases. At the baseline visit, 74% of the overall cohort received no treatment, while 19% underwent local treatment and only 2% underwent systemic treatment. Of the local therapies, anti-VEGF injections were the most frequently performed procedure (33%, mainly for secondary CNV), followed by micropulse laser (28%), focal nonpulsed laser (23%), verteporfin photodynamic therapy (14%), and nonsteroidal anti-inflammatory eye drops (2%). Among intravitreal anti-VEGF agents, aflibercept was used most frequently, followed by bevacizumab and ranibizumab.
Conclusion: This registry represents one of the largest cohorts of European patients with CSC to date. Patient age and the proportion of women were higher than expected and bilateral active disease was lower than anticipated, highlighting that neither age nor gender should be overemphasized when diagnosing CSC. Therapeutic interventions are heterogeneous and include verteporfin photodynamic therapy, micropulse laser, and anti-VEGF injections in case of secondary CNV.
Keywords: Central serous chorioretinopathy; Demographics; Incidence; Registry; Retina.net; Treatment.
© 2024 The Author(s). Published by S. Karger AG, Basel.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Figures




References
-
- Von Graefe A. Über zentrale rezidivierende Retinitis. Graefes Arch Clin Exp Ophthalmol. 1866;12:221.
-
- Lange C, Treumer F, Bertram B, Feltgen N, Hoerauf H, Pauleikhoff D, et al. . Chorioretinopathia centralis serosa (CCS). Stellungnahme der Deutschen Ophthalmologischen Gesellschaft, der Retinologischen Gesellschaft und des Berufsverbandes der Augenärzte Deutschlands. Der Ophthalmologe: Zeitschrift Der Deutschen Ophthalmologischen Gesellschaft; 2022. print; last updated.10.2021.
-
- Pauleikhoff L, Agostini H, Lange C. [Central serous chorioretinopathy]. Ophthalmologe. 2021;118(9):967–80. - PubMed
-
- van Rijssen TJ, van Dijk EHC, Yzer S, Ohno-Matsui K, Keunen JEE, Schlingemann RO, et al. . Central serous chorioretinopathy: towards an evidence-based treatment guideline. Prog Retin Eye Res. 2019;73:100770. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous

