Effect of a multi-strain probiotic mixture consumption on anxiety and depression symptoms induced in adult mice by postnatal maternal separation
- PMID: 38369490
- PMCID: PMC10875865
- DOI: 10.1186/s40168-024-01752-w
Effect of a multi-strain probiotic mixture consumption on anxiety and depression symptoms induced in adult mice by postnatal maternal separation
Abstract
Background: Intestinal microbial composition not only affects the health of the gut but also influences centrally mediated systems involved in mood, through the "gut-brain" axis, a bidirectional communication between gut microbiota and the brain. In this context, the modulation of intestinal microbiota and its metabolites through the administration of probiotics seems to represent a very promising approach in the treatment of the central nervous system alterations. Early postnatal life is a critical period during which the brain undergoes profound and essential modulations in terms of maturation and plasticity. Maternal separation (MS), i.e., the disruption of the mother-pup interaction, represents a pivotal paradigm in the study of stress-related mood disorders, by inducing persistent changes in the immune system, inflammatory processes, and emotional behavior in adult mammals.
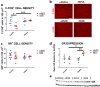
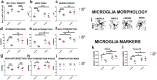
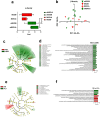
Results: We conducted experiments to investigate whether sustained consumption of a multi-strain probiotic formulation by adult male mice could mitigate the effects of maternal separation. Our data demonstrated that the treatment with probiotics was able to totally reverse the anxiety- and depressive-like behavior; normalize the neuro-inflammatory state, by restoring the resting state of microglia; and finally induce a proneurogenic effect. Mice subjected to maternal separation showed changes in microbiota composition compared to the control group that resulted in permissive colonization by the administered multi-strain probiotic product. As a consequence, the probiotic treatment also significantly affected the production of SCFA and in particular the level of butyrate.
Conclusion: Gut microbiota and its metabolites mediate the therapeutic action of the probiotic mix on MS-induced brain dysfunctions. Our findings extend the knowledge on the use of probiotics as a therapeutic tool in the presence of alterations of the emotional sphere that significantly impact on gut microbiota composition. Video Abstract.
Keywords: Adult neurogenesis; Anxiety and depression; Gut-brain axis; Inflammation; Microbiota; Probiotic; Short-chain fatty acids.
© 2024. The Author(s).
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper. Prof. Diego Mora sits on the Scientific Advisory Board of Actial Farmaceutica Srl, and has provided, as a part of his Institutional Activity, technical reports for this company and Beingpharma Srl.
Figures



References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials

