The oncolytic bacteria-mediated delivery system of CCDC25 nucleic acid drug inhibits neutrophil extracellular traps induced tumor metastasis
- PMID: 38369519
- PMCID: PMC10875894
- DOI: 10.1186/s12951-024-02335-5
The oncolytic bacteria-mediated delivery system of CCDC25 nucleic acid drug inhibits neutrophil extracellular traps induced tumor metastasis
Abstract
Background: Neutrophil extracellular traps (NETs), antibacterial weapons of neutrophils (NEs), have been found to play a crucial role in cancer metastasis in recent years. More and more cancer research is focusing on anti-NETs. However, almost all anti-NETs treatments have limitations such as large side effects and limited efficacy. Therefore, exploring new anti-NETs therapeutic strategies is a long-term goal.
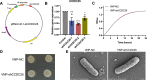
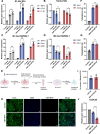
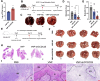
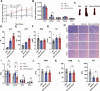
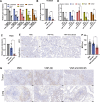
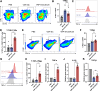
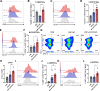
Results: The transmembrane protein coiled-coil domain containing 25 (CCDC25) on tumor cell membranes can bind NETs-DNA with high specificity and affinity, enabling tumor cells to sense NETs and thus promote distant metastasis. We transformed shCCDC25 into VNP20009 (VNP), an oncolytic bacterium, to generate VNP-shCCDC25 and performed preclinical evaluation of the inhibitory effect of shCCDC25 on cancer metastasis in B16F10 lung metastasis and 4T1 orthotopic lung metastasis models. VNP-shCCDC25 effectively blocked the downstream prometastatic signaling pathway of CCDC25 at tumor sites and reduced the formation of NETs while recruiting more neutrophils and macrophages to the tumor core, ultimately leading to excellent metastasis inhibition in the two lung metastasis models.
Conclusion: This study is a pioneer in focusing on the effect of anti-NET treatment on CCDC25. shCCDC25 is effectively delivered to tumor sites via the help of oncolytic bacteria and has broad application in the inhibition of cancer metastasis via anti-NETs.
Keywords: CCDC25; Metastasis; NETs; Neutrophils; Nucleic acid delivery; VNP20009.
© 2024. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

References
MeSH terms
Substances
Grants and funding
- 82130106/the National Natural Sciences Foundation of China
- 32250016/the National Natural Sciences Foundation of China
- 202110016, China/Nanjing Special Fund for Life and Health Science and Technology
- CJ20210024/Changzhou Bureau of Science and Technology
- CZ20210010/Changzhou Bureau of Science and Technology
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

