Toxoflavin analog D43 exerts antiproliferative effects on breast cancer by inducing ROS-mediated apoptosis and DNA damage
- PMID: 38369538
- PMCID: PMC10874970
- DOI: 10.1038/s41598-024-53843-1
Toxoflavin analog D43 exerts antiproliferative effects on breast cancer by inducing ROS-mediated apoptosis and DNA damage
Abstract
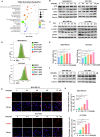
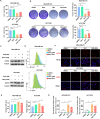
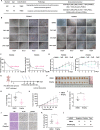
Triple-negative breast cancer (TNBC) is regarded as the deadliest subtype of breast cancer because of its high heterogeneity, aggressiveness, and limited treatment options. Toxoflavin has been reported to possess antitumor activity. In this study, a series of toxoflavin analogs were synthesized, among which D43 displayed a significant dose-dependent inhibitory effect on the proliferation of TNBC cells (MDA-MB-231 and HCC1806). Additionally, D43 inhibited DNA synthesis in TNBC cells, leading to cell cycle arrest at the G2/M phase. Furthermore, D43 consistently promoted intracellular ROS generation, induced DNA damage, and resulted in apoptosis in TNBC cells. These effects could be reversed by N-acetylcysteine. Moreover, D43 significantly inhibited the growth of breast cancer patient-derived organoids and xenografts with a favorable biosafety profile. In conclusion, D43 is a potent anticancer agent that elicits significant antiproliferation, oxidative stress, apoptosis, and DNA damage effects in TNBC cells, and D43 holds promise as a potential candidate for the treatment of TNBC.
Keywords: DNA damage; N-acetylcysteine (NAC); Patient-derived breast cancer organoids (PDO); ROS; Toxoflavin.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

