Bioactive glass-ceramics containing fluorapatite, xonotlite, cuspidine and wollastonite form apatite faster than their corresponding glasses
- PMID: 38369547
- PMCID: PMC10874964
- DOI: 10.1038/s41598-024-54228-0
Bioactive glass-ceramics containing fluorapatite, xonotlite, cuspidine and wollastonite form apatite faster than their corresponding glasses
Abstract
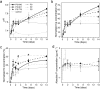
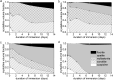
Crystallisation of bioactive glasses has been claimed to negatively affect the ion release from bioactive glasses. Here, we compare ion release and mineralisation in Tris-HCl buffer solution for a series of glass-ceramics and their parent glasses in the system SiO2-CaO-P2O5-CaF2. Time-resolved X-ray diffraction analysis of glass-ceramic degradation, including quantification of crystal fractions by full pattern refinement, show that the glass-ceramics precipitated apatite faster than the corresponding glasses, in agreement with faster ion release from the glass-ceramics. Imaging by transmission electron microscopy and X-ray nano-computed tomography suggest that this accelerated degradation may be caused by the presence of nano-sized channels along the internal crystal/glassy matrix interfaces. In addition, the presence of crystalline fluorapatite in the glass-ceramics facilitated apatite nucleation and crystallisation during immersion. These results suggest that the popular view of bioactive glass crystallisation being a disadvantage for degradation, apatite formation and, subsequently, bioactivity may depend on the actual system study and, thus, has to be reconsidered.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures





References
-
- Hench LL, Day DE, Höland W, Rheinberger VM. Glass and medicine. Int. J. Appl. Glass Sci. 2010;1:104–117. doi: 10.1111/j.2041-1294.2010.00001.x. - DOI
-
- Jones JR, Boccaccini AR. Cellular Ceramics: Structure, Manufacturing, Processing and Applications. Wiley; 2005. pp. 547–570.
Grants and funding
LinkOut - more resources
Full Text Sources

