Seroprevalence of poliovirus antibodies in Nigeria: refining strategies to sustain the eradication effort
- PMID: 38370104
- PMCID: PMC10874096
- DOI: 10.11604/pamj.supp.2023.45.2.38098
Seroprevalence of poliovirus antibodies in Nigeria: refining strategies to sustain the eradication effort
Abstract
Introduction: in 2016, a switch from trivalent oral poliovirus vaccine (OPV) (containing serotypes 1,2,3) to bivalent OPV (types 1,3) was implemented globally. We assessed the seroprevalence of poliovirus antibody levels in selected Nigerian states, before and after the switch, documented poliovirus type2 outbreak responses conducted and ascertained factors associated with immunity gaps based on seroprevalence rates.
Methods: we conducted a secondary analysis of stored serum samples from the 2018 Nigeria National HIV/AIDS Indicator and Impact Survey. Serum from 1,185 children aged 0-119 months residing in one southern and four northern states were tested for serotype-specific PV neutralizing antibodies; seropositivity was a reciprocal titer ≥8. We conducted regression analysis to determine sociodemographic risk factors associated with low seroprevalence using SAS 9.4.
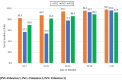
Results: children aged 24-119 months (pre-switch cohort) had seroprevalence against PV1, PV2, and PV3, of 97.3% (95% CI:96.4-98.2), 93.8% (95% CI:92.2-95.5), and 91.3% (95% CI:89.2-93.4), while children aged <24 months (post-switch) had seroprevalence of 86.0% (95% CI:81.2-90.8), 55.6% (95% CI: 47.7-63.4), and 77.2% (95% CI:71.0-83.4) respectively. Regression analysis showed age <24 months was associated with lower seroprevalence against all PV serotypes, (p<0.0001); females had lower seroprevalence against PV1 (p=0.0184) and PV2 (p=0.0354); northern states lower seroprevalence against PV1 (p=0.0039), while well-water source lower seroprevalence against PV3 (p=0.0288).
Conclusion: this study showed high seroprevalence rates against PV 1, 2, and 3 in pre-switch children (aged 24-119 months). However, post-switch children (<24 months) had low immunity against PV2 despite outbreak responses. Strategies to increase routine immunization coverage and high-quality polio campaigns can increase immunity against polio virus.
Keywords: Nigeria; Prevalence; antibodies; disease outbreaks; immunity; immunization; polio virus vaccine; poliomyelitis; risk factors; serotype.
©Omotayo Bolu et al.
Conflict of interest statement
The authors declare no competing interests.
Figures
References
-
- World Health Organization Global eradication of poliomyelitis by the year 2000: The Forty-first World Health Assembly. May 13 1988, Accessed September 21, 2021.
-
- Global Polio Eradication Initiative . Declaration Further Milestone for Globally-Coordinated Vaccine Switch in 2016. Global eradication of wild poliovirus type 2 declared. Accessed September 21, 2021.
-
- Kuehn B. Poliovirus type 3 is eradicated. JAMA. 2019 Dec 17;322(23):2276. - PubMed
-
- Guglielmi G. Africa declared free from wild polio - but vaccine-derived strains remain. Nature. 2020. Aug 28, - PubMed
-
- Hampton LM, Farrell M, Ramirez-Gonzalez A, Menning L, Shendale S, Lewis I, et al. Cessation of Trivalent Oral Poliovirus Vaccine and Introduction of Inactivated Poliovirus Vaccine-Worldwide, 2016. MMWR Morb Mortal Wkly Rep. 2016 Sep 9;65(35):934–8. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical