Deployment of novel oral polio vaccine type 2 under emergency use listing in Nigeria: the rollout experience
- PMID: 38370105
- PMCID: PMC10874098
- DOI: 10.11604/pamj.supp.2023.45.2.38033
Deployment of novel oral polio vaccine type 2 under emergency use listing in Nigeria: the rollout experience
Abstract
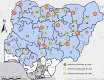
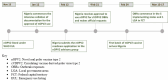
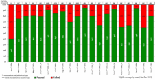
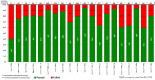
In 2011, a dedicated consortium of experts commenced work on the development of the novel oral poliovirus vaccine type 2 (nOPV2). After careful and rigorous analysis of data to enable early, targeted use of the vaccine, World Health Organization´s (WHO´s) Strategic Advisory Group of Experts on Immunization (SAGE) reviewed data from accelerated clinical development of nOPV2 and endorsed entering assessment under WHO´s Emergency Use Listing (EUL) procedure. In November 2020, nOPV2 received an interim recommendation for use under EUL to enable rapid field availability and potential wider rollout of the vaccine. In December 2020, Nigeria initiated preparation to meet all criteria for initial use of nOPV2 in the country and the documentation process to verify meeting them. The process entailed addressing the status of meeting 25 readiness criteria in nine categories for nOPV2 use in Nigeria for response efforts to ongoing cVDPV2 outbreaks. During January-February 2021, Nigeria submitted the required documentation for all required indicators for nOPV2 initial use. In February 2021, the country obtained approval from the GPEI nOPV2 Readiness Verification Team to introduce nOPV2 and in March 2021, rolled out the novel vaccine in mass vaccination campaigns for outbreak response in Bayelsa, Delta, Niger, Sokoto and Zamfara states, and one area council in the Federal Capital Territory (FCT). The lessons learned from this rollout experience in Nigeria are being applied as the country streamlines and strengthens the nOPV2 rollout process across the remaining states.
Keywords: Nigeria; Prevalence; antibodies; disease outbreaks; immunity; immunization; oral; polio virus vaccine; poliomyelitis; risk factors; serotype.
©Adeyelu Asekun et al.
Conflict of interest statement
The authors declare no competing interests.
Figures
References
-
- Rotary World Polio Day cheers major achievements toward global polio eradication. 2019. Accessed Sep 8, 2021.
-
- World Health Organization Africa eradicates wild poliovirus. August 25 2020, Accessed Sep 8, 2021.
-
- Global Polio Eradication Initiative Circulating vaccine-derived poliovirus. March 28 2021, Accessed Sep 8 2021.
-
- World Health Organization Weekly GPEI Polio Analyses. Accessed Sep 8 2022.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
