Oral β-Lactams, Fluoroquinolones, or Trimethoprim-Sulfamethoxazole for Definitive Treatment of Uncomplicated Escherichia coli or Klebsiella Species Bacteremia From a Urinary Tract Source
- PMID: 38370295
- PMCID: PMC10873539
- DOI: 10.1093/ofid/ofad657
Oral β-Lactams, Fluoroquinolones, or Trimethoprim-Sulfamethoxazole for Definitive Treatment of Uncomplicated Escherichia coli or Klebsiella Species Bacteremia From a Urinary Tract Source
Erratum in
-
Correction to: Oral β-Lactams, Fluoroquinolones, or Trimethoprim-Sulfamethoxazole for Definitive Treatment of Uncomplicated Escherichia coli or Klebsiella Species Bacteremia From a Urinary Tract Source.Open Forum Infect Dis. 2024 Apr 9;11(4):ofae191. doi: 10.1093/ofid/ofae191. eCollection 2024 Apr. Open Forum Infect Dis. 2024. PMID: 38595958 Free PMC article.
Abstract
Background: Fluoroquinolones (FQs) are effective for oral step-down therapy for gram-negative bloodstream infections but are associated with unfavorable toxic effects. Robust data are lacking for trimethoprim-sulfamethoxazole (TMP-SMX) and high-bioavailability β-lactams (HBBLs).
Methods: In this multicenter observational cohort study, we simulated a 3-arm registry trial using causal inference methods to compare the effectiveness of FQs, TMP-SMX, or HBBLs for gram-negative bloodstream infections oral step-down therapy. The study included adults treated between January 2016 and December 2022 for uncomplicated Escherichia coli or Klebsiella species bacteremia of urinary tract origin who were who were transitioned to an oral regimen after ≤4 days of effective intravenous antibiotics. Propensity weighting was used to balance characteristics between groups. 60-day recurrence was compared using a multinomial Cox proportional hazards model with probability of treatment weighting.
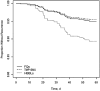
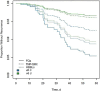
Results: Of 2571 patients screened, 648 (25%) were included. Their median age (interquartile range) was 67 (45-78) years, and only 103 (16%) were male. Characteristics were well balanced between groups. Compared with FQs, TMP-SMX had similar effectiveness (adjusted hazard ratio, 0.91 [95% confidence interval, .30-2.78]), and HBBLs had a higher risk of recurrence (2.19 [.95-5.01]), although this difference was not statistically significant. Most HBBLs (70%) were not optimally dosed for bacteremia. A total antibiotic duration ≤8 days was associated with a higher recurrence rate in select patients with risk factors for failure.
Conclusions: FQs and TMP-SMX had similar effectiveness in this real-world data set. HBBLs were associated with higher recurrence rates but suboptimal dosing may have contributed. Further studies are needed to define optimal BL dosing and duration to mitigate treatment failures.
Keywords: antimicrobial stewardship; gram-negative bacteremia; real-world evidence; urinary tract infection; β-lactams.
© The Author(s) 2023. Published by Oxford University Press on behalf of Infectious Diseases Society of America.
Conflict of interest statement
Potential conflicts of interest. The authors: No reported conflicts of interest.
Figures

References
-
- Tamma PD, Cosgrove SE. Which trial do we need? early oral antibiotic therapy for the treatment of gram-negative bloodstream infections. Clin Microbiol Infect 2023; 29:670–2. - PubMed
-
- Sandberg T, Skoog G, Hermansson AB, et al. Ciprofloxacin for 7 days versus 14 days in women with acute pyelonephritis: a randomised, open-label and double-blind, placebo-controlled, non-inferiority trial. Lancet 2012; 380:484–90. - PubMed

