HCV-induced autophagy and innate immunity
- PMID: 38370419
- PMCID: PMC10874285
- DOI: 10.3389/fimmu.2024.1305157
HCV-induced autophagy and innate immunity
Abstract
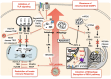
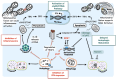
The interplay between autophagy and host innate immunity has been of great interest. Hepatitis C virus (HCV) impedes signaling pathways initiated by pattern-recognition receptors (PRRs) that recognize pathogens-associated molecular patterns (PAMPs). Autophagy, a cellular catabolic process, delivers damaged organelles and protein aggregates to lysosomes for degradation and recycling. Autophagy is also an innate immune response of cells to trap pathogens in membrane vesicles for removal. However, HCV controls the autophagic pathway and uses autophagic membranes to enhance its replication. Mitophagy, a selective autophagy targeting mitochondria, alters the dynamics and metabolism of mitochondria, which play important roles in host antiviral responses. HCV also alters mitochondrial dynamics and promotes mitophagy to prevent premature cell death and attenuate the interferon (IFN) response. In addition, the dysregulation of the inflammasomal response by HCV leads to IFN resistance and immune tolerance. These immune evasion properties of HCV allow HCV to successfully replicate and persist in its host cells. In this article, we discuss HCV-induced autophagy/mitophagy and its associated immunological responses and provide a review of our current understanding of how these processes are regulated in HCV-infected cells.
Keywords: HCV; STING; autophagy; inflammasome; interferons; mitophagy; oxidative stress.
Copyright © 2024 Lee and Ou.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Hepatitis C virus and intracellular antiviral response.Curr Opin Virol. 2022 Feb;52:244-249. doi: 10.1016/j.coviro.2021.12.010. Epub 2021 Dec 29. Curr Opin Virol. 2022. PMID: 34973476 Free PMC article. Review.
-
Autophagy in HCV Replication and Protein Trafficking.Int J Mol Sci. 2021 Jan 22;22(3):1089. doi: 10.3390/ijms22031089. Int J Mol Sci. 2021. PMID: 33499186 Free PMC article. Review.
-
Hepatitis C Virus-Induced Autophagy and Host Innate Immune Response.Viruses. 2017 Aug 12;9(8):224. doi: 10.3390/v9080224. Viruses. 2017. PMID: 28805674 Free PMC article. Review.
-
Hepatitis C virus and autophagy.Biol Chem. 2015 Nov;396(11):1215-22. doi: 10.1515/hsz-2015-0172. Biol Chem. 2015. PMID: 26024249 Free PMC article. Review.
-
Activation of the unfolded protein response and autophagy after hepatitis C virus infection suppresses innate antiviral immunity in vitro.J Clin Invest. 2011 Jan;121(1):37-56. doi: 10.1172/JCI41474. Epub 2010 Dec 6. J Clin Invest. 2011. PMID: 21135505 Free PMC article.
Cited by
-
Getah virus triggers ROS-mediated autophagy in mouse Leydig cells.Front Microbiol. 2025 Jan 13;15:1519694. doi: 10.3389/fmicb.2024.1519694. eCollection 2024. Front Microbiol. 2025. PMID: 39872815 Free PMC article.
-
Crosstalk between Dysfunctional Mitochondria and Proinflammatory Responses during Viral Infections.Int J Mol Sci. 2024 Aug 24;25(17):9206. doi: 10.3390/ijms25179206. Int J Mol Sci. 2024. PMID: 39273156 Free PMC article. Review.
-
The Emerging Roles of Vacuolar-Type ATPase-Dependent Lysosomal Acidification in Cardiovascular Disease.Biomolecules. 2025 Apr 3;15(4):525. doi: 10.3390/biom15040525. Biomolecules. 2025. PMID: 40305271 Free PMC article. Review.
-
Vital Role of PINK1/Parkin-Mediated Mitophagy of Pulmonary Epithelial Cells in Severe Pneumonia Induced by IAV and Secondary Staphylococcus aureus Infection.Int J Mol Sci. 2025 Apr 27;26(9):4162. doi: 10.3390/ijms26094162. Int J Mol Sci. 2025. PMID: 40362402 Free PMC article.
-
Role of mitochondria in physiological activities, diseases, and therapy.Mol Biomed. 2025 Jun 19;6(1):42. doi: 10.1186/s43556-025-00284-5. Mol Biomed. 2025. PMID: 40536597 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

