Solvatochromism of new tetraphenylethene luminogens: integration of aggregation-induced emission and conjugation-induced rigidity for emitting strongly in both solid and solution state
- PMID: 38370453
- PMCID: PMC10870197
- DOI: 10.1039/d4ra00719k
Solvatochromism of new tetraphenylethene luminogens: integration of aggregation-induced emission and conjugation-induced rigidity for emitting strongly in both solid and solution state
Abstract
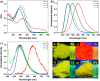
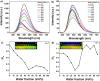
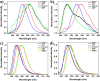
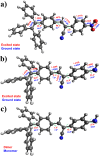
In this study, we synthesized and characterized four tetraphenylethene (TPE) analogs, investigated their photophysical properties, and conducted quantum chemical calculations. Some molecules exhibited aggregation-induced emission enhancement behavior and showed efficient emission in both solid and solution states. Solvatochromism was observed in particular derivatives, with solvent polarity influencing either a bathochromic or hypsochromic shift, indicating the occurrence of photoinduced intramolecular charge transfer (ICT) processes. Quantum chemical calculations confirmed that variations in molecular packing and rigidity among the TPE analogs contributed to their diverse behavior. The study showcases aggregation in luminophores without significant impact on the excited state and highlights how minor alterations in terminal substituents can lead to unconventional behavior. These findings have implications for the development of luminescent materials. Furthermore, the synthesized compounds exhibited biocompatibility, suggesting their potential for cell imaging applications.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures




References
-
- Grimsdale A. C. Chan K. L. Martin R. E. Jokisz P. G. Holmes A. B. Synthesis of light-emitting conjugated polymers for applications in electroluminescent devices. Chem. Rev. 2009;109:897–1091. doi: 10.1021/cr000013v. https://dx.doi.org/10.1021/cr000013v - DOI - DOI - PubMed
-
- Müllen K. K., Scherf U. and John Wiley & Sons., Organic Light Emitting Devices: Synthesis, Properties and Applications, Wiley-VCH, 2006. https://www.wiley.com/en-ae/Organic+Light+Emitting+Devices%3A+Synthesis%..., accessed June 4, 2019
-
- Anthony J. E. Functionalized Acenes and Heteroacenes for Organic Electronics. Chem. Rev. 2006;106:5028–5048. doi: 10.1021/cr050966z. https://dx.doi.org/10.1021/CR050966Z - DOI - DOI - PubMed
-
- Kometani N. Nakajima H. Asami K. Yonezawa Y. Scheblykin I. G. Vitukhnovsky A. G. Luminescence properties of the J-aggregate of cyanine dyes in multilayer assemblies. J. Lumin. 2000;87–89:770–772. doi: 10.1016/S0022-2313(99)00395-6. https://dx.doi.org/10.1016/S0022-2313(99)00395-6 - DOI - DOI
-
- Lu H. Wang Y. Hill S. K. Jiang H. Ke Y. Huang S. Zheng D. Perrier S. Song Q. Supra-Cyanines: Ultrabright Cyanine-Based Fluorescent Supramolecular Materials in Solution and in the Solid State. Angew. Chem., Int. Ed. 2023;62:e202311224. doi: 10.1002/anie.202311224. https://dx.doi.org/10.1002/anie.202311224 - DOI - DOI - PubMed
LinkOut - more resources
Full Text Sources

