This is a preprint.
TNF promoter hypomethylation is associated with mucosal inflammation in IBD and anti-TNF response
- PMID: 38370739
- PMCID: PMC10871362
- DOI: 10.1101/2024.02.05.24302343
TNF promoter hypomethylation is associated with mucosal inflammation in IBD and anti-TNF response
Update in
-
TNF Promoter Hypomethylation Is Associated With Mucosal Inflammation in IBD and Anti-TNF Response.Gastro Hep Adv. 2024 Jun 27;3(7):888-898. doi: 10.1016/j.gastha.2024.06.010. eCollection 2024. Gastro Hep Adv. 2024. PMID: 39286616 Free PMC article.
Abstract
Background and aims: Inflammatory Bowel Diseases (IBD) are chronic inflammatory conditions influenced heavily by environmental factors. DNA methylation is a form of epigenetic regulation linking environmental stimuli to gene expression changes and inflammation. Here, we investigated how DNA methylation of the TNF promoter differs between inflamed and uninflamed mucosa of IBD patients, including anti-TNF responders and non-responders.
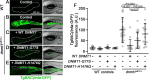
Methods: We obtained mucosal biopsies from 200 participants (133 IBD and 67 controls) and analyzed TNF promoter methylation using bisulfite sequencing, comparing inflamed with uninflamed segments, in addition to paired inflamed/uninflamed samples from individual patients. We conducted similar analyses on purified intestinal epithelial cells from bowel resections. We also compared TNF methylation levels of inflamed and uninflamed mucosa from a separate cohort of 15 anti-TNF responders and 17 non-responders. Finally, we sequenced DNA methyltransferase genes to identify rare variants in IBD patients and functionally tested them using rescue experiments in a zebrafish genetic model of DNA methylation deficiency.
Results: TNF promoter methylation levels were decreased in inflamed mucosa of IBD patients and correlated with disease severity. Isolated IECs from inflamed tissue showed proportional decreases in TNF methylation. Anti-TNF non-responders showed lower levels of TNF methylation than responders in uninflamed mucosa. Our sequencing analysis revealed two missense variants in DNMT1, one of which had reduced function in vivo.
Conclusions: Our study reveals an association of TNF promoter hypomethylation with mucosal inflammation, suggesting that IBD patients may be particularly sensitive to inflammatory environmental insults affecting DNA methylation. Together, our analyses indicate that TNF promoter methylation analysis may aid in the characterization of IBD status and evaluation of anti-TNF therapy response.
Keywords: Anit-TNF Response; Epigenetics; Inflammatory Bowel Disease; Methylation.
Conflict of interest statement
Disclosures The authors have no conflicts of interest to disclose.
Figures


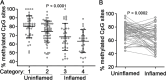


References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
