This is a preprint.
De novo mutations mediate phenotypic switching in an opportunistic human lung pathogen
- PMID: 38370793
- PMCID: PMC10871308
- DOI: 10.1101/2024.02.06.579193
De novo mutations mediate phenotypic switching in an opportunistic human lung pathogen
Update in
-
De novo mutations mediate phenotypic switching in an opportunistic human lung pathogen.Nat Commun. 2025 Jul 23;16(1):6799. doi: 10.1038/s41467-025-61168-4. Nat Commun. 2025. PMID: 40701980 Free PMC article.
Abstract
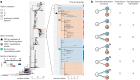
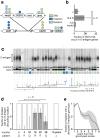
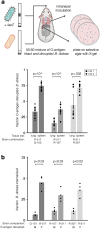
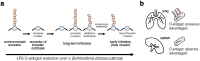
Bacteria evolving within human hosts encounter selective tradeoffs that render mutations adaptive in one context and deleterious in another. Here, we report that the cystic fibrosis-associated pathogen Burkholderia dolosa overcomes in-human selective tradeoffs by acquiring successive point mutations that alternate phenotypes. We sequenced the whole genomes of 931 respiratory isolates from two recently infected patients and an epidemiologically-linked, chronically-infected patient. These isolates are contextualized using 112 historical genomes from the same outbreak strain. Within both newly infected patients, diverse parallel mutations that disrupt O-antigen expression quickly arose, comprising 29% and 63% of their B. dolosa communities by 3 years. The selection for loss of O-antigen starkly contrasts with our previous observation of parallel O-antigen-restoring mutations after many years of chronic infection in the historical outbreak. Experimental characterization revealed that O-antigen loss increases uptake in immune cells while decreasing competitiveness in the mouse lung. We propose that the balance of these pressures, and thus whether O-antigen expression is advantageous, depends on tissue localization and infection duration. These results suggest that mutation-driven alternation during infection may be more frequent than appreciated and is underestimated without dense temporal sampling.
Figures

References
-
- Ferenci T. Trade-off Mechanisms Shaping the Diversity of Bacteria. Trends Microbiol. 24, 209–223 (2016). - PubMed
-
- Cooper V. S. & Lenski R. E. The population genetics of ecological specialization in evolving Escherichia coli populations. Nature 407, 736–739 (2000). - PubMed
-
- Juhas M. Horizontal gene transfer in human pathogens. Crit. Rev. Microbiol. 41, 101–108 (2015). - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
