Solid-Phase Synthesis as a Tool to Create Exactly Defined, Branched Polymer Vectors for Cell Membrane Targeting
- PMID: 38370914
- PMCID: PMC10867888
- DOI: 10.1021/acs.macromol.3c02600
Solid-Phase Synthesis as a Tool to Create Exactly Defined, Branched Polymer Vectors for Cell Membrane Targeting
Abstract
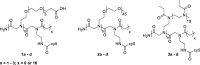
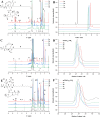
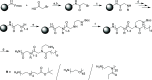
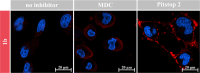
Modern drug formulations often require, besides the active drug molecule, auxiliaries to enhance their pharmacological properties. Tailor-made, biocompatible polymers covalently connected to the drug molecule can fulfill this function by increasing its solubility, reducing its toxicity, and guiding it to a specific target. If targeting membrane-bound proteins, localization of the drug close to the cell membrane and its target is beneficial to increase drug efficiency and residence time. In this study, we present the synthesis of highly defined, branched polymeric structures with membrane-binding properties. One to three hydrophilic poly(ethylene oxide) or poly(2-ethyloxazoline) side chains were connected via a peptoid backbone using a two-step iterative protocol for solid-phase peptoid synthesis. Additional groups, e.g., a hydrophobic anchor for membrane attachment, were introduced. Due to the nature of solid-phase synthesis, the number and order of the side chains and additional units can be precisely defined. The method proved to be versatile for the generation of multifunctional, branched polymeric structures of molecular weights up to approximately 7000 g mol-1. The behavior of all compounds towards biological membranes and cells was investigated using liposomes as cell membrane models, HEK293 and U251-MG cell lines, and red blood cells, thereby demonstrating their potential value as drug auxiliaries with cell membrane affinity.
© 2024 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
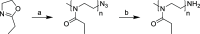




Similar articles
-
Solid-phase submonomer synthesis of peptoid polymers and their self-assembly into highly-ordered nanosheets.J Vis Exp. 2011 Nov 2;(57):e3373. doi: 10.3791/3373. J Vis Exp. 2011. PMID: 22083233 Free PMC article.
-
Erratum: Preparation of Poly(pentafluorophenyl acrylate) Functionalized SiO2 Beads for Protein Purification.J Vis Exp. 2019 Apr 30;(146). doi: 10.3791/6328. J Vis Exp. 2019. PMID: 31038480
-
Poly(peptide): Synthesis, Structure, and Function of Peptide-Polymer Amphiphiles and Protein-like Polymers.Acc Chem Res. 2020 Feb 18;53(2):400-413. doi: 10.1021/acs.accounts.9b00518. Epub 2020 Jan 22. Acc Chem Res. 2020. PMID: 31967781 Free PMC article.
-
Polymeric vesicles: from drug carriers to nanoreactors and artificial organelles.Acc Chem Res. 2011 Oct 18;44(10):1039-49. doi: 10.1021/ar200036k. Epub 2011 May 24. Acc Chem Res. 2011. PMID: 21608994 Review.
-
'Smart' delivery systems for biomolecular therapeutics.Orthod Craniofac Res. 2005 Aug;8(3):219-25. doi: 10.1111/j.1601-6343.2005.00336.x. Orthod Craniofac Res. 2005. PMID: 16022724 Review.
Cited by
-
On-Resin Recycling of Acid-Labile Linker Enables the Reuse of Solid Support for Fmoc-Based Solid Phase Synthesis.Macromol Rapid Commun. 2025 Aug;46(15):e2500073. doi: 10.1002/marc.202500073. Epub 2025 Mar 8. Macromol Rapid Commun. 2025. PMID: 40056078 Free PMC article.
-
Solid Lipid Nanoparticles Coated with Glucosylated poly(2-oxazoline)s: A Supramolecular Toolbox Approach.Biomacromolecules. 2025 Feb 10;26(2):861-882. doi: 10.1021/acs.biomac.4c01052. Epub 2025 Jan 8. Biomacromolecules. 2025. PMID: 39779305 Free PMC article.
-
Peptide discovery across the spectrum of neuroinflammation; microglia and astrocyte phenotypical targeting, mediation, and mechanistic understanding.Front Mol Neurosci. 2024 Nov 20;17:1443985. doi: 10.3389/fnmol.2024.1443985. eCollection 2024. Front Mol Neurosci. 2024. PMID: 39634607 Free PMC article. Review.
References
-
- Nishiyama N.; Okazaki S.; Cabral H.; Miyamoto M.; Kato Y.; Sugiyama Y.; Nishio K.; Matsumura Y.; Kataoka K. Novel Cisplatin-Incorporated Polymeric Micelles Can Eradicate Solid Tumors in Mice. Cancer Res. 2003, 63 (24), 8977–8983. - PubMed
LinkOut - more resources
Full Text Sources
