Adjuvant Osimertinib in Patients With Stage IB to IIIA EGFR Mutation-Positive NSCLC After Complete Tumor Resection: ADAURA China Subgroup Analysis
- PMID: 38371194
- PMCID: PMC10874739
- DOI: 10.1016/j.jtocrr.2023.100621
Adjuvant Osimertinib in Patients With Stage IB to IIIA EGFR Mutation-Positive NSCLC After Complete Tumor Resection: ADAURA China Subgroup Analysis
Abstract
Introduction: In Chinese patients with NSCLC, prevalence of EGFR-mutated (EGFRm) disease is high. In the global phase 3 ADAURA study (NCT02511106), adjuvant osimertinib was found to have a statistically significant and clinically meaningful improvement in disease-free survival (DFS) versus placebo in resected stage IB to IIIA EGFRm NSCLC. We present efficacy and safety data from a subgroup analysis of 159 Chinese patients enrolled in the People's Republic of China from ADAURA.
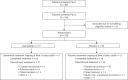
Methods: In ADAURA, patients with completely resected stage IB to IIIA EGFRm (exon 19 deletion/exon 21 L858R) NSCLC were randomized 1:1 to receive osimertinib (80 mg once daily) or placebo for 3 years or until disease recurrence/discontinuation. Adjuvant chemotherapy was permitted before randomization, per physician/patient choice. Primary end point was investigator-assessed DFS in stage II to IIIA disease; secondary end points included DFS in stage IB to IIIA (overall population), overall survival, health-related quality of life (HRQoL), and safety.
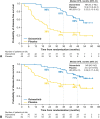
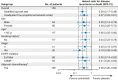
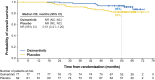
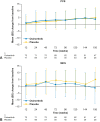
Results: Of 682 patients enrolled globally, 159 patients in the People's Republic of China were included in this subgroup analysis (osimertinib n = 77; placebo n = 82). Baseline characteristics were balanced across the treatment arms. At data cutoff, stage II to IIIA DFS hazard ratio (HR) was 0.23 (95% confidence interval [CI]: 0.13-0.42; maturity 59%); stage IB to IIIA DFS HR was 0.29 (95% CI: 0.17-0.48; maturity 42%). At 13% maturity (21 deaths), HR for overall survival in the stage IB to IIIA population was 0.51 (95% CI: 0.21-1.20). HRQoL was maintained from baseline, and safety was consistent with the global population.
Conclusions: In this population of Chinese patients from ADAURA, adjuvant osimertinib was found to have a clinically meaningful improvement in DFS versus placebo, with maintained HRQoL and a safety profile consistent with the global study population.
Keywords: Adjuvant; China; EGFR; NSCLC; Osimertinib.
© 2024 Published by Elsevier Inc. on behalf of the International Association for the Study of Lung Cancer.
Figures
Similar articles
-
Adjuvant Osimertinib for Resected EGFR-Mutated Stage IB-IIIA Non-Small-Cell Lung Cancer: Updated Results From the Phase III Randomized ADAURA Trial.J Clin Oncol. 2023 Apr 1;41(10):1830-1840. doi: 10.1200/JCO.22.02186. Epub 2023 Jan 31. J Clin Oncol. 2023. PMID: 36720083 Free PMC article. Clinical Trial.
-
Postoperative Chemotherapy Use and Outcomes From ADAURA: Osimertinib as Adjuvant Therapy for Resected EGFR-Mutated NSCLC.J Thorac Oncol. 2022 Mar;17(3):423-433. doi: 10.1016/j.jtho.2021.10.014. Epub 2021 Nov 2. J Thorac Oncol. 2022. PMID: 34740861 Clinical Trial.
-
Osimertinib: A Review in Completely Resected, Early-Stage, EGFR Mutation-Positive NSCLC.Target Oncol. 2022 May;17(3):369-376. doi: 10.1007/s11523-022-00883-0. Epub 2022 Jun 17. Target Oncol. 2022. PMID: 35713772 Review.
-
Deconstructing ADAURA: It is Time to Forgo Adjuvant Platinum-Based Chemotherapy in Resected IB-IIIA EGFR+ NSCLC (Except with RB Alterations?) When Adopting Adjuvant Osimertinib.Lung Cancer (Auckl). 2022 Apr 26;13:23-31. doi: 10.2147/LCTT.S358902. eCollection 2022. Lung Cancer (Auckl). 2022. PMID: 35506019 Free PMC article.
-
The dawn of a new era, adjuvant EGFR inhibition in resected non-small cell lung cancer.Ther Adv Med Oncol. 2021 Nov 15;13:17588359211056306. doi: 10.1177/17588359211056306. eCollection 2021. Ther Adv Med Oncol. 2021. PMID: 34804219 Free PMC article. Review.
Cited by
-
Drug development and evidence for lung cancer targeted therapy in Eastern Asia.Lancet Reg Health West Pac. 2024 Jul 8;49:101090. doi: 10.1016/j.lanwpc.2024.101090. eCollection 2024 Aug. Lancet Reg Health West Pac. 2024. PMID: 39381018 Free PMC article. Review.
-
Adjuvant epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors (TKIs) for the treatment of people with resected stage I to III non-small-cell lung cancer and EGFR mutation.Cochrane Database Syst Rev. 2025 May 27;5(5):CD015140. doi: 10.1002/14651858.CD015140.pub2. Cochrane Database Syst Rev. 2025. PMID: 40421698 Review.
-
Targeting metastatic cancer.Nat Med. 2021 Jan;27(1):34-44. doi: 10.1038/s41591-020-01195-4. Epub 2021 Jan 13. Nat Med. 2021. PMID: 33442008 Free PMC article. Review.
-
Non-small cell lung cancer in China.Cancer Commun (Lond). 2022 Oct;42(10):937-970. doi: 10.1002/cac2.12359. Epub 2022 Sep 8. Cancer Commun (Lond). 2022. PMID: 36075878 Free PMC article. Review.
References
-
- Remon J., Soria J.C., Peters S., ESMO Guidelines Committee Early and locally advanced non-small-cell lung cancer: an update of the ESMO Clinical Practice Guidelines focusing on diagnosis, staging, systemic and local therapy. Ann Oncol. 2021;32:1637–1642. - PubMed
-
- Kris M.G., Gaspar L.E., Chaft J.E., et al. Adjuvant systemic therapy and adjuvant radiation therapy for stage I to IIIA completely resected non-small-cell lung cancers: American Society of Clinical Oncology/Cancer Care Ontario Clinical Practice Guideline Update. J Clin Oncol. 2017;35:2960–2974. - PubMed
-
- Postmus P.E., Kerr K.M., Oudkerk M., et al. Early and locally advanced non-small-cell lung cancer (NSCLC): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2017;28(suppl 4):iv1–iv21. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

