Antimicrobial Peptide-Poly(ethylene glycol) Conjugates: Connecting Molecular Architecture, Solution Properties, and Functional Performance
- PMID: 38371733
- PMCID: PMC10870750
- DOI: 10.1021/acspolymersau.3c00026
Antimicrobial Peptide-Poly(ethylene glycol) Conjugates: Connecting Molecular Architecture, Solution Properties, and Functional Performance
Abstract
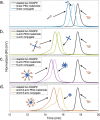
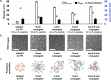
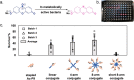
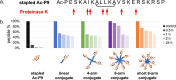
Antimicrobial peptides (AMPs) are promising alternatives to conventional antibiotics for treating infections caused by drug-resistant bacteria; yet, many peptides are limited by toxicity to eukaryotic cells and instability in biological environments. Conjugation to linear polymers that reduce cytotoxicity and improve stability, however, often decreases antimicrobial activity. In this work, we combine the biocompatibility advantages of poly(ethylene glycol) (PEG) with the efficacy merits of nonlinear polymer architectures that accommodate multiple AMPs per molecule. By conjugating a chemokine-derived AMP, stapled Ac-P9, to linear and star-shaped PEG with various arm numbers and lengths, we investigated the role of molecular architecture in solution properties (i.e., ζ-potential, size, and morphology) and performance (i.e., antimicrobial activity, hemolysis, and protease resistance). Linear, 4-arm, and 8-arm conjugates with 2-2.5 kDa PEG arms were found to form nanoscale structures in solution with lower ζ-potentials relative to the unconjugated AMP, suggesting that the polymer partially shields the cationic AMP. Reducing the length of the PEG arms of the 8-arm conjugate to 1.25 kDa appeared to better reveal the peptide, seen by the increased ζ-potential, and promote assembly into particles with a larger size and defined spherical morphology. The antimicrobial effects exerted by the short 8-arm conjugate rivaled that of the unconjugated peptide, and the AMP constituents of the short 8-arm conjugate were protected from proteolytic degradation. All other conjugates examined also imparted a degree of protease resistance, but exhibited some reduced level of antimicrobial activity as compared to the AMP alone. None of the conjugates caused significant cytotoxic effects, which bodes well for their future potential to treat infections. While enhancing proteolytic stability often comes with the cost of lower antimicrobial activity, we have found that presenting AMPs at high density on a neutral nonlinear polymer strikes a favorable balance, exhibiting both enhanced stability and high antimicrobial activity.
© 2023 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

References
-
- O’Neill J.Tackling Drug-Resistant Infections Globally: Final Report and Recommendations 2016https://amr-review.org/sites/default/files/160518_Final%20paper_with%20c... (accessed November 20, 2023).
Grants and funding
LinkOut - more resources
Full Text Sources
