Strategy and Technical Progress of Recycling of Spent Vanadium-Titanium-Based Selective Catalytic Reduction Catalysts
- PMID: 38371753
- PMCID: PMC10870271
- DOI: 10.1021/acsomega.3c07019
Strategy and Technical Progress of Recycling of Spent Vanadium-Titanium-Based Selective Catalytic Reduction Catalysts
Abstract
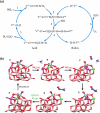
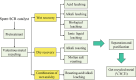
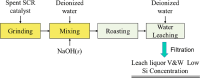
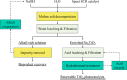
Selective catalytic reduction denitration technology, abbreviated as SCR, is essential for the removal of nitrogen oxide from the flue gas of coal-fired power stations and has been widely used. Due to the strong demand for energy and the requirements for environmental protection, a large amount of SCR catalyst waste is produced. The spent SCR catalyst contains high-grade valuable metals, and proper disposal or treatment of the SCR catalyst can protect the environment and realize resource recycling. This review focuses on the two main routes of regeneration and recycling of spent vanadium-titanium SCR catalysts that are currently most widely commercially used and summarizes in detail the technologies of recycling, high-efficiency recycling, and recycling of valuable components of spent vanadium-titanium SCR catalysts. This review also discusses in depth the future development direction of recycling spent vanadium-titanium SCR catalysts. It provides a reference for promoting recycling, which is crucial for resource recovery and green and low-carbon development.
© 2024 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
















Similar articles
-
A comprehensive review of the heavy metal issues regarding commercial vanadium‑titanium-based SCR catalyst.Sci Total Environ. 2023 Jan 20;857(Pt 3):159712. doi: 10.1016/j.scitotenv.2022.159712. Epub 2022 Oct 25. Sci Total Environ. 2023. PMID: 36302402 Review.
-
Progress of selective catalytic reduction denitrification catalysts at wide temperature in carbon neutralization.Front Chem. 2022 Aug 17;10:946133. doi: 10.3389/fchem.2022.946133. eCollection 2022. Front Chem. 2022. PMID: 36059869 Free PMC article. Review.
-
A Review on Resource Utilization of Spent V-W-Ti Based Selective Catalytic Reduction Catalysts.Materials (Basel). 2022 Nov 11;15(22):7984. doi: 10.3390/ma15227984. Materials (Basel). 2022. PMID: 36431471 Free PMC article. Review.
-
Recent trends in vanadium-based SCR catalysts for NOx reduction in industrial applications: stationary sources.Nano Converg. 2022 Nov 19;9(1):51. doi: 10.1186/s40580-022-00341-7. Nano Converg. 2022. PMID: 36401645 Free PMC article. Review.
-
Deactivation Mechanism of Spent TiO2-Based Selective Catalytic Reduction (SCR) Catalysts and State-of-the-Art Recycling Technologies.Langmuir. 2024 Dec 17;40(50):26371-26386. doi: 10.1021/acs.langmuir.4c03515. Epub 2024 Dec 4. Langmuir. 2024. PMID: 39630120 Review.
References
-
- Chen X.; Geng Y.; Shan W.; Liu F. Deactivation Effects of Potassium on a CeMoTiOx Catalyst for the Selective Catalytic Reduction of NOx with NH3. Ind. Eng. Chem. Res. 2018, 57, 1399–1407. 10.1021/acs.iecr.7b04444. - DOI
-
- Liu Z.; Han J.; Zhao L.; Wu Y.-w.; Wang H.-x.; Pei X.-q.; Xu M.-x.; Lu Q.; Yang Y.-p. Effects of Se and SeO2 on the denitrification performance of V2O5-WO3/TiO2 SCR catalyst. Appl. Catal. A Gen. 2019, 587, 117263.10.1016/j.apcata.2019.117263. - DOI
-
- Schill L.; Fehrmann R. Strategies of coping with deactivation of NH3-SCR catalysts due to biomass firing. Catalysts 2018, 8, 135.10.3390/catal8040135. - DOI
Publication types
LinkOut - more resources
Full Text Sources
