A novel NLRP3 inhibitor as a therapeutic agent against monosodium urate-induced gout
- PMID: 38371945
- PMCID: PMC10869544
- DOI: 10.3389/fimmu.2023.1307739
A novel NLRP3 inhibitor as a therapeutic agent against monosodium urate-induced gout
Abstract
Background: Since NEK7 is critical for NLRP3 inflammasome activation, NEK7 inhibitors could be employed as therapeutic agents against gout, a representative disease caused by NLRP3 inflammasome.
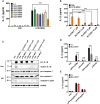
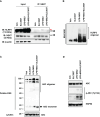
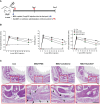
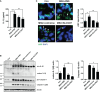
Methods: We designed NEK7 inhibitors based on biochemical kinome profiling of 2,7-substituted thieno[3,2-d]pyrimidine derivatives (SLC3031~3035 and SLC3037). Inflammasome activation was assessed by ELISA of IL-1b and immunoblotting of IL-1b maturation after treatment of bone marrow-derived macrophages with LPS+monosodium urate (MSU). NLPR3 binding to NEK7 and oligomerization were examined using immunoprecipitation and Blue Native gel electrophoresis, respectively. In vivo effect was investigated by studying gross and histopathological changes of food pad tissue of MSU-injected mice, together with assays of maturation of IL-1b and ASC speck in the tissue.
Results: SLC3037 inhibited inflammasome by MSU and other inflammasome activators through blockade of NLRP3 binding to NEK7 or oligomerization, and subsequent ASC oligomerization/phosphorylation. SLC3037 significantly reduced foot pad thickness and inflammation by MSU, which was superior to the effects of colchicine. SLC3037 significantly reduced content or maturation of IL-1b and ASC speck in the food pad. The number and height of intestinal villi were decreased by colchicine but not by SLC3037.
Conclusion: SLC3037, a NLRP3 inhibitor blocking NEK7 binding to NLRP3, could be a novel agent against diseases associated with NLRP3 inflammasome activation such as gout, cardiovascular diseases, metabolic syndrome or neurodegenerative diseases.
Keywords: NEK7; crystal; gout; inflammasome; monosodium urate.
Copyright © 2024 Park, Shin, Kim, Kang, Oh, Jang, Sim, Youn and Lee.
Conflict of interest statement
M-SL is the CEO of LysoTech, Inc. TS is a shareholder of Magicbullet therapeutics Inc. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

