Multiple approaches to reduce reconstitution time of lyophilized drug products with high protein concentration
- PMID: 38371955
- PMCID: PMC10873283
- DOI: 10.1093/abt/tbad031
Multiple approaches to reduce reconstitution time of lyophilized drug products with high protein concentration
Abstract
Background: Lyophilized drug products with high protein concentration often perform long reconstitution time, which is inconvenient for clinical use. The objective of this work is to achieve short reconstitution time with multiple and combined strategies.
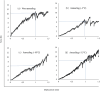
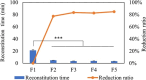
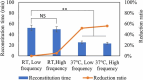
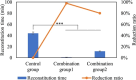
Methods: Here, we describe the following approaches that lead to reduction of reconstitution time, including adding annealing step, decreasing headspace pressure, decreasing protein concentration with reducing diluent volume, increasing high surface-area-to-height ratio of the cakes, increasing frequency of swirling and diluent temperature.
Results: Among these strategies, reducing diluent volume to achieve high protein concentration and reducing headspace pressure show markedly reduction of reconstitution time. Moreover, we propose combined strategies to mitigate the reconstitution time, at the same time, to achieve same target dose in clinics.
Conclusions: Therefore, this paper provides insights on the application of multiple strategies to accelerate the reconstitution of lyophilized drug products with high concentration, and facilitates their widespread clinical application.
Keywords: combined strategy; headspace pressure; lyophilized drug products with high concentration; multiple approaches; reconstitution time; reducing diluent volume.
© The Author(s) 2023. Published by Oxford University Press on behalf of Antibody Therapeutics.
Conflict of interest statement
Xiaozhang Zhang, Ningning Zhou, Chunsheng Yang, Zhaowei Jin and Jeremy Guo are employees of Drug Product Development, Shang Hai WuXi Biologics Inc.
Figures




References
-
- Bhambhani, A, Blue, JT. Lyophilization strategies for development of a high-concentration monoclonal antibody formulation: benefits and pitfalls. Am Pharm Rev 2010; 13: 31–8.
-
- Shire, SJ, Shahrokh, Z, Liu, J. Challenges in the development of high protein concentration formulations. J Pharm Sci 2004; 93: 1390–402. - PubMed
-
- Cao, W, Krishnan, S, Ricci, MSet al. . Rational design of lyophilized high concentration protein formulations-mitigating the challenge of slow reconstitution with multidisciplinary strategies. Eur J Pharm Biopharm 2013; 85: 287–93. - PubMed
LinkOut - more resources
Full Text Sources
