Tetraether archaeal lipids promote long-term survival in extreme conditions
- PMID: 38372181
- PMCID: PMC11096074
- DOI: 10.1111/mmi.15240
Tetraether archaeal lipids promote long-term survival in extreme conditions
Abstract
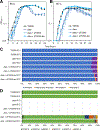
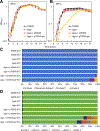
The sole unifying feature of the incredibly diverse Archaea is their isoprenoid-based ether-linked lipid membranes. Unique lipid membrane composition, including an abundance of membrane-spanning tetraether lipids, impart resistance to extreme conditions. Many questions remain, however, regarding the synthesis and modification of tetraether lipids and how dynamic changes to archaeal lipid membrane composition support hyperthermophily. Tetraether membranes, termed glycerol dibiphytanyl glycerol tetraethers (GDGTs), are generated by tetraether synthase (Tes) by joining the tails of two bilayer lipids known as archaeol. GDGTs are often further specialized through the addition of cyclopentane rings by GDGT ring synthase (Grs). A positive correlation between relative GDGT abundance and entry into stationary phase growth has been observed, but the physiological impact of inhibiting GDGT synthesis has not previously been reported. Here, we demonstrate that the model hyperthermophile Thermococcus kodakarensis remains viable when Tes (TK2145) or Grs (TK0167) are deleted, permitting phenotypic and lipid analyses at different temperatures. The absence of cyclopentane rings in GDGTs does not impact growth in T. kodakarensis, but an overabundance of rings due to ectopic Grs expression is highly fitness negative at supra-optimal temperatures. In contrast, deletion of Tes resulted in the loss of all GDGTs, cyclization of archaeol, and loss of viability upon transition to the stationary phase in this model archaea. These results demonstrate the critical roles of highly specialized, dynamic, isoprenoid-based lipid membranes for archaeal survival at high temperatures.
Keywords: GDGT; archaea; archaeol; tetraether lipids; tetraether synthase; thermophily.
© 2024 The Authors. Molecular Microbiology published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare that there is no conflict of interest.
Figures


Similar articles
-
Identification of two archaeal GDGT lipid-modifying proteins reveals diverse microbes capable of GMGT biosynthesis and modification.Proc Natl Acad Sci U S A. 2024 Jun 25;121(26):e2318761121. doi: 10.1073/pnas.2318761121. Epub 2024 Jun 17. Proc Natl Acad Sci U S A. 2024. PMID: 38885389 Free PMC article.
-
Identification of a protein responsible for the synthesis of archaeal membrane-spanning GDGT lipids.Nat Commun. 2022 Mar 22;13(1):1545. doi: 10.1038/s41467-022-29264-x. Nat Commun. 2022. PMID: 35318330 Free PMC article.
-
GDGT cyclization proteins identify the dominant archaeal sources of tetraether lipids in the ocean.Proc Natl Acad Sci U S A. 2019 Nov 5;116(45):22505-22511. doi: 10.1073/pnas.1909306116. Epub 2019 Oct 7. Proc Natl Acad Sci U S A. 2019. PMID: 31591189 Free PMC article.
-
Archeal Di-O-geranylgeranyl Glyceryl Phosphate Synthase of a UbiA Superfamily Member Provides Insight into the Multiple Human Diseases.Protein Pept Lett. 2020;27(7):568-573. doi: 10.2174/0929866526666191209143948. Protein Pept Lett. 2020. PMID: 31814543 Review.
-
Biosynthesis of archaeal membrane ether lipids.Front Microbiol. 2014 Nov 26;5:641. doi: 10.3389/fmicb.2014.00641. eCollection 2014. Front Microbiol. 2014. PMID: 25505460 Free PMC article. Review.
Cited by
-
Archaeal Lipids: Extraction, Separation, and Identification via Natural Product Chemistry Perspective.Int J Mol Sci. 2025 Mar 29;26(7):3167. doi: 10.3390/ijms26073167. Int J Mol Sci. 2025. PMID: 40243902 Free PMC article. Review.
-
Engineering archaeal membrane-spanning lipid GDGT biosynthesis in bacteria: Implications for early life membrane transformations.mLife. 2025 Mar 13;4(2):193-204. doi: 10.1002/mlf2.70001. eCollection 2025 Apr. mLife. 2025. PMID: 40313982 Free PMC article.
-
Identification of two archaeal GDGT lipid-modifying proteins reveals diverse microbes capable of GMGT biosynthesis and modification.Proc Natl Acad Sci U S A. 2024 Jun 25;121(26):e2318761121. doi: 10.1073/pnas.2318761121. Epub 2024 Jun 17. Proc Natl Acad Sci U S A. 2024. PMID: 38885389 Free PMC article.
-
Bilayer-Forming Lipids Enhance Archaeal Monolayer Membrane Stability.Int J Mol Sci. 2025 Mar 26;26(7):3045. doi: 10.3390/ijms26073045. Int J Mol Sci. 2025. PMID: 40243703 Free PMC article.
-
Cyclization of archaeal membrane lipids impacts membrane protein activity and archaellum formation.Proc Natl Acad Sci U S A. 2025 May 20;122(20):e2423648122. doi: 10.1073/pnas.2423648122. Epub 2025 May 12. Proc Natl Acad Sci U S A. 2025. PMID: 40354536
References
-
- Dawson KS, Freeman KH, & Macalady JL (2012). Molecular characterization of core lipids from halophilic archaea grown under different salinity conditions. Organic Geochemistry, 48, 1–8. 10.1016/J.ORGGEOCHEM.2012.04.003 - DOI
-
- Elling FJ, Könneke M, Lipp JS, Becker KW, Gagen EJ, & Hinrichs KU (2014). Effects of growth phase on the membrane lipid composition of the thaumarchaeon Nitrosopumilus maritimus and their implications for archaeal lipid distributions in the marine environment. Geochimica et Cosmochimica Acta, 141, 579–597. 10.1016/J.GCA.2014.07.005 - DOI
-
- Hurley SJ, Elling FJ, Könneke M, Buchwald C, Wankel SD, Santoro AE, Lipp JS, Hinrichs KU, & Pearson A (2016). Influence of ammonia oxidation rate on thaumarchaeal lipid composition and the TEX86 temperature proxy. Proceedings of the National Academy of Sciences of the United States of America, 113(28), 7762–7767. 10.1073/PNAS.1518534113/SUPPL_FILE/PNAS.1518534113.SAPP.PDF - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

