Unearthing the role of septins in viral infections
- PMID: 38372298
- PMCID: PMC10920062
- DOI: 10.1042/BSR20231827
Unearthing the role of septins in viral infections
Abstract
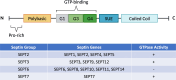
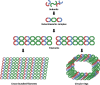
Septin proteins are a subfamily of closely related GTP-binding proteins conserved in all species except for higher plants and perform essential biological processes. Septins self-assemble into heptameric or octameric complexes and form higher-order structures such as filaments, rings, or gauzes by end-to-end binding. Their close association with cell membrane components makes them central in regulating critical cellular processes. Due to their organisation and properties, septins function as diffusion barriers and are integral in providing scaffolding to support the membrane's curvature and stability of its components. Septins are also involved in vesicle transport and exocytosis through the plasma membrane by co-localising with exocyst protein complexes. Recently, there have been emerging reports of several human and animal diseases linked to septins and abnormalities in their functions. Most of our understanding of the significance of septins during microbial diseases mainly pertains to their roles in bacterial infections but not viruses. This present review focuses on the known roles of septins in host-viral interactions as detailed by various studies.
Keywords: diseases; host-viral interaction; roles; septin.
© 2024 The Author(s).
Conflict of interest statement
The authors declare that there are no competing interests associated with the manuscript.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

