Durability of Protection Against COVID-19 Through the Delta Surge for the NVX-CoV2373 Vaccine
- PMID: 38372392
- PMCID: PMC11259228
- DOI: 10.1093/cid/ciae081
Durability of Protection Against COVID-19 Through the Delta Surge for the NVX-CoV2373 Vaccine
Abstract
Background: Protein-based vaccines for coronavirus disease 2019 (COVID-19) provide a traditional vaccine platform with long-lasting protection for non-severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pathogens and may complement messenger RNA vaccines as a booster dose. While NVX-CoV2373 showed substantial early efficacy, the durability of protection has not been delineated.
Methods: The PREVENT-19 vaccine trial used a blinded crossover design; the original placebo arm received NVX-CoV2373 after efficacy was established. Using novel statistical methods that integrate surveillance data of circulating strains with post-crossover cases, we estimated placebo-controlled vaccine efficacy and durability of NVX-CoV2373 against both pre-Delta and Delta strains of SARS-CoV-2.
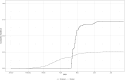
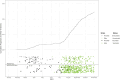
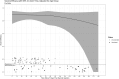
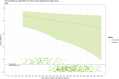
Results: Vaccine efficacy against pre-Delta strains of COVID-19 was 89% (95% CI, 75-95%) and 87% (72-94%) at 0 and 90 days after 2 doses of NVX-CoV2373, respectively, with no evidence of waning (P = .93). Vaccine efficacy against the Delta strain was 88% (71-95%), 82% (56-92%), and 77% (44-90%) at 40, 120, and 180 days, respectively, with evidence of waning (P < .01). In sensitivity analyses, the estimated Delta vaccine efficacy at 120 days ranged from 66% (15-86%) to 89% (74-95%) per various assumptions of the surveillance data.
Conclusions: NVX-CoV2373 has high initial efficacy against pre-Delta and Delta strains of COVID-19 with little evidence of waning for pre-Delta strains through 90 days and moderate waning against Delta strains over 180 days.
Keywords: COVID-19; NVX-CoV2373; SARS-CoV-2; vaccine durability.
Published by Oxford University Press on behalf of Infectious Diseases Society of America 2024.
Conflict of interest statement
Potential conflicts of interest. M. P. F. reports support for meetings and/or travel from NIAID. A. M. received funds from the National Cancer Institute. C. A. M. received funds to their institution from the National Institutes of Health Coronavirus Prevention Network. Y. H., H. A., and K. K. received funds to their institution from NIAID. Y. H. received funds to their institution from the World Health Organization. K. K. also received funds to their institution for Novavax: A 2-Part Phase 2/3 Open-Label Study to Evaluate the Safety and Immunogenicity of a XBB.1.5 (Omicron Subvariant) SARS-CoV-2 rS Vaccine Booster Dose in Previously mRNA COVID-19 Vaccinated and Baseline SARS-CoV-2 Seropositive COVID-19 Vaccine Naïve Participants and has leadership as the National Co-Chair Novavax Phase 3 Trials of NIAID. L. M. D., W. W., and I. C. are employees and stockholders of Novavax, Inc. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Figures
Similar articles
-
Immunogenicity and safety following a homologous booster dose of a SARS-CoV-2 recombinant spike protein vaccine with Matrix-MTM adjuvant (NVX-CoV2373) versus a primary series in people living with and without HIV-1 infection in South Africa: A randomized crossover phase 2a/2b trial.Hum Vaccin Immunother. 2024 Dec 31;20(1):2425147. doi: 10.1080/21645515.2024.2425147. Epub 2024 Dec 12. Hum Vaccin Immunother. 2024. PMID: 39666396 Free PMC article. Clinical Trial.
-
Safety and immunogenicity following a homologous booster dose of a SARS-CoV-2 recombinant spike protein vaccine (NVX-CoV2373): a secondary analysis of a randomised, placebo-controlled, phase 2 trial.Lancet Infect Dis. 2022 Nov;22(11):1565-1576. doi: 10.1016/S1473-3099(22)00420-0. Epub 2022 Aug 10. Lancet Infect Dis. 2022. PMID: 35963274 Free PMC article. Clinical Trial.
-
Safety, Immunogenicity, and Efficacy of the NVX-CoV2373 COVID-19 Vaccine in Adolescents: A Randomized Clinical Trial.JAMA Netw Open. 2023 Apr 3;6(4):e239135. doi: 10.1001/jamanetworkopen.2023.9135. JAMA Netw Open. 2023. PMID: 37099299 Free PMC article. Clinical Trial.
-
Safety, efficacy, and immunogenicity of the NVX-CoV2373 vaccine.Expert Rev Vaccines. 2023 Jan-Dec;22(1):501-517. doi: 10.1080/14760584.2023.2218913. Expert Rev Vaccines. 2023. PMID: 37246757 Review.
-
The Novavax Heterologous Coronavirus Disease 2019 Booster Demonstrates Lower Reactogenicity Than Messenger RNA: A Targeted Review.J Infect Dis. 2024 Aug 16;230(2):e496-e502. doi: 10.1093/infdis/jiad519. J Infect Dis. 2024. PMID: 37992183 Free PMC article. Review.
Cited by
-
Cost-Effectiveness of Introducing Nuvaxovid to COVID-19 Vaccination in the United Kingdom: A Dynamic Transmission Model.Vaccines (Basel). 2025 Feb 14;13(2):187. doi: 10.3390/vaccines13020187. Vaccines (Basel). 2025. PMID: 40006733 Free PMC article.
-
Safety and immunogenicity of four sequential doses of NVX-CoV2373 in adults and adolescents: A phase 3, randomized, placebo-controlled trial (PREVENT-19).Vaccine. 2025 Jun 5;61:127362. doi: 10.1016/j.vaccine.2025.127362. Online ahead of print. Vaccine. 2025. PMID: 40479932
References
-
- Centers for Disease Control and Prevention . Variants of the virus. Available at: https://www.cdc.gov/coronavirus/2019-ncov/variants/index.html. Accessed 1 November 2023.
-
- Couzin-Frankel J. Should you pick Novavax’s COVID-19 shot over mRNA options? Science 2023; 382:141–2. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
- Medical CBRN
- National Institute of Allergy and Infectious Diseases
- Biomedical Advanced Research and Development Authority
- W911QY20C0077/Department of Defense
- UM1 AI068618/AI/NIAID NIH HHS/United States
- UM1 AI148684/AI/NIAID NIH HHS/United States
- UM1 AI068635/AI/NIAID NIH HHS/United States
- Novavax
- UM1 AI68618/HVTN Laboratory Center
- UM1 AI68619/HIV Prevention Trials Network Leadership and Operations Center
- UM1 AI068636/AI/NIAID NIH HHS/United States
- UM1 AI148689/AI/NIAID NIH HHS/United States
- UM1 AI68636/AIDS Clinical Trials Group Leadership and Operations Center
- UM1 AI68635/HVTN Statistics and Data Management Center
- CA/NCI NIH HHS/United States
- NH/NIH HHS/United States
- UM1 AI068619/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

