Critical Role of CD55 in Controlling Wound Healing
- PMID: 38372645
- PMCID: PMC12005244
- DOI: 10.4049/jimmunol.2300628
Critical Role of CD55 in Controlling Wound Healing
Abstract
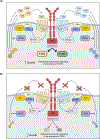
How reparative processes are coordinated following injury is incompletely understood. In recent studies, we showed that autocrine C3a and C5a receptor (C3ar1 and C5ar1) G protein-coupled receptor signaling plays an obligate role in vascular endothelial growth factor receptor 2 growth signaling in vascular endothelial cells. We documented the same interconnection for platelet-derived growth factor receptor growth signaling in smooth muscle cells, epidermal growth factor receptor growth signaling in epidermal cells, and fibroblast growth factor receptor signaling in fibroblasts, indicative of a generalized cell growth regulatory mechanism. In this study, we examined one physiological consequence of this signaling circuit. We found that disabling CD55 (also known as decay accelerating factor), which lifts restraint on autocrine C3ar1/C5ar1 signaling, concomitantly augments the growth of each cell type. The mechanism is heightened C3ar1/C5ar1 signaling resulting from the loss of CD55's restraint jointly potentiating growth factor production by each cell type. Examination of the effect of lifted CD55 restraint in four types of injury (burn, corneal denudation, ear lobe puncture, and reengraftment of autologous skin) showed that disabled CD55 function robustly accelerated healing in all cases, whereas disabled C3ar1/C5ar1 signaling universally retarded it. In wild-type mice with burns or injured corneas, applying a mouse anti-mouse CD55 blocking Ab (against CD55's active site) to wounds accelerated the healing rate by 40-70%. To our knowledge, these results provide new insights into mechanisms that underlie wound repair and open up a new tool for accelerating healing.
Copyright © 2024 by The American Association of Immunologists, Inc.
Conflict of interest statement
STAEMENTS AND DECLARATIONS
The authors do not have any competing financial or non-financial interests.
The data have not been previously published elsewhere.
Figures




References
-
- Hong YK, Lange-Asschenfeldt B, Velasco P, Hirakawa S, Kunstfeld R, Brown LF, Bohlen P, Senger DR, and Detmar M 2004. VEGF-A promotes tissue repair-associated lymphatic vessel formation via VEGFR-2 and the alpha1beta1 and alpha2beta1 integrins. FASEB journal : official publication of the Federation of American Societies for Experimental Biology 18: 1111–1113. - PubMed
-
- Strainic MG, Pohlmann E, Valley CC, Sammeta A, Hussain W, Lidke DS, and Medof ME 2020. RTK signaling requires C3ar1/C5ar1 and IL-6R joint signaling to repress dominant PTEN, SOCS1/3 and PHLPP restraint. FASEB journal : official publication of the Federation of American Societies for Experimental Biology 34: 2105–2125. - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

