Immune-related serious adverse events with immune checkpoint inhibitors: Systematic review and network meta-analysis
- PMID: 38372756
- PMCID: PMC11001692
- DOI: 10.1007/s00228-024-03647-z
Immune-related serious adverse events with immune checkpoint inhibitors: Systematic review and network meta-analysis
Erratum in
-
Correction to: Immune‑related serious adverse events with immune checkpoint inhibitors: systematic review and network meta‑analysis.Eur J Clin Pharmacol. 2024 Oct;80(10):1597-1598. doi: 10.1007/s00228-024-03732-3. Eur J Clin Pharmacol. 2024. PMID: 39043974 Free PMC article. No abstract available.
Abstract
Purpose: Immune checkpoint inhibitors (ICIs) have revolutionized cancer treatment, though uncertainty exists regarding their immune-related safety. The objective of this study was to assess the comparative safety profile (odds ratio) of ICIs and estimate the absolute rate of immune-related serious adverse events (irSAEs) in cancer patients undergoing treatment with ICIs.
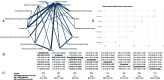
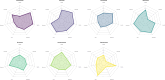
Methods: We searched for randomized trials till February 2021, including all ICIs for all cancers. Primary outcome was overall irSAEs, and secondary outcomes were pneumonitis, colitis, hepatitis, hypophysitis, myocarditis, nephritis, and pancreatitis. We conducted Bayesian network meta-analyses, estimated absolute rates and ranked treatments according to the surface under the cumulative ranking curve (SUCRA).
Results: We included 96 trials (52,811 participants, median age 62 years). Risk of bias was high in most trials. Most cancers were non-small cell lung cancer (28 trials) and melanoma (15 trials). The worst-ranked ICI was ipilimumab (SUCRA 14%; event rate 848/10,000 patients) while the best-ranked ICI was atezolizumab (SUCRA 82%; event rate 119/10,000 patients).
Conclusion: Each ICI showed a unique safety profile, with certain events more frequently observed with specific ICIs, which should be considered when managing cancer patients.
Keywords: Cancer; Clinical trials; Immune check-point inhibitors; Network meta-analysis; Safety; Systematic review.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
References
-
- Puzanov I, Diab A, Abdallah K et al (2017) Managing toxicities associated with immune checkpoint inhibitors: consensus recommendations from the Society for Immunotherapy of Cancer (SITC) Toxici-ty Management Working Group. J Immunother Cancer 5:1–28. 10.1186/s40425-017-0300-z 10.1186/s40425-017-0300-z - DOI - PMC - PubMed
-
- Khoja L, Day D, Wei-Wu Chen T et al (2017) Tumour- and class-specific patterns of immune-related adverse events of immune check-point inhibitors: A systematic review 28:2377–2385 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

