Propyl gallate induces human pulmonary fibroblast cell death through the regulation of Bax and caspase-3
- PMID: 38373208
- PMCID: PMC10878342
- DOI: 10.1080/07853890.2024.2319853
Propyl gallate induces human pulmonary fibroblast cell death through the regulation of Bax and caspase-3
Abstract
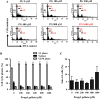
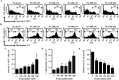
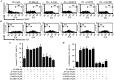
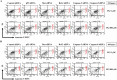
Propyl gallate (PG) has been found to exert an inhibitory effect on the growth of different cell types, including lung cancer cells. However, little is known about the cytotoxicological effects of PG specifically on normal primary lung cells. The current study examined the cellular effects and cell death resulting from PG treatment in human pulmonary fibroblast (HPF) cells. DNA flow cytometry results demonstrated that PG (100-1,600 μM) had a significant impact on the cell cycle, leading to G1 phase arrest. Notably, 1,600 μM PG slightly increased the number of sub-G1 cells. Additionally, PG (400-1,600 μM) resulted in the initiation of cell death, a process that coincided with a loss of mitochondrial membrane potential (MMP; ΔΨm). This loss of MMP (ΔΨm) was evaluated using a FACS cytometer. In PG-treated HPF cells, inhibitors targeting pan-caspase, caspase-3, caspase-8, and caspase-9 showed no significant impact on the quantity of annexin V-positive and MMP (ΔΨm) loss cells. The administration of siRNA targeting Bax or caspase-3 demonstrated a significant attenuation of PG-induced cell death in HPF cells. However, the use of siRNAs targeting p53, Bcl-2, or caspase-8 did not exhibit any notable effect on cell death. Furthermore, none of the tested MAPK inhibitors, including MEK, c-Jun N-terminal kinase (JNK), and p38, showed any impact on PG-induced cell death or the loss of MMP (ΔΨm) in HPF cells. In conclusion, PG induces G1 phase arrest of the cell cycle and cell death in HPF cells through apoptosis and/or necrosis. The observed HPF cell death is mediated by the modulation of Bax and caspase-3. These findings offer insights into the cytotoxic and molecular effects of PG on normal HPF cells.
Keywords: Human pulmonary fibroblast; caspase; cell cycle; cell death; mitogen-activated protein kinase; propyl gallate.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
Similar articles
-
Propyl gallate induces cell death in human pulmonary fibroblast through increasing reactive oxygen species levels and depleting glutathione.Sci Rep. 2024 Mar 5;14(1):5375. doi: 10.1038/s41598-024-52849-z. Sci Rep. 2024. PMID: 38438412 Free PMC article.
-
Enhanced cell death effects of MAP kinase inhibitors in propyl gallate-treated lung cancer cells are related to increased ROS levels and GSH depletion.Toxicol In Vitro. 2021 Aug;74:105176. doi: 10.1016/j.tiv.2021.105176. Epub 2021 Apr 16. Toxicol In Vitro. 2021. PMID: 33865947
-
Propyl gallate reduces the growth of lung cancer cells through caspase‑dependent apoptosis and G1 phase arrest of the cell cycle.Oncol Rep. 2020 Dec;44(6):2783-2791. doi: 10.3892/or.2020.7815. Epub 2020 Oct 20. Oncol Rep. 2020. PMID: 33125113
-
Proteasome inhibition by MG132 induces growth inhibition and death of human pulmonary fibroblast cells in a caspase-independent manner.Oncol Rep. 2011 Jun;25(6):1705-12. doi: 10.3892/or.2011.1211. Epub 2011 Mar 8. Oncol Rep. 2011. PMID: 21399877
-
Propyl gallate inhibits the growth of HeLa cells via caspase-dependent apoptosis as well as a G1 phase arrest of the cell cycle.Oncol Rep. 2010 Apr;23(4):1153-8. doi: 10.3892/or_00000745. Oncol Rep. 2010. PMID: 20204304
Cited by
-
Mechanism of Valeriana officinalis L. extract improving atherosclerosis by regulating PGC-1α/Sirt3/Epac1 pathway.Front Pharmacol. 2024 Nov 19;15:1483518. doi: 10.3389/fphar.2024.1483518. eCollection 2024. Front Pharmacol. 2024. PMID: 39629078 Free PMC article.
References
-
- Final report on the amended safety assessment of propyl gallate. Int J Toxicol. 2007;26(Suppl 3):1–12. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
