Treatment Satisfaction With Omnipod DASH in Adults With Type 1 Diabetes: A Nonblinded 1:1 Randomized Controlled Trial
- PMID: 38373265
- PMCID: PMC11244188
- DOI: 10.1210/clinem/dgae088
Treatment Satisfaction With Omnipod DASH in Adults With Type 1 Diabetes: A Nonblinded 1:1 Randomized Controlled Trial
Abstract
Context: Omnipod DASH Insulin Management System is a tubeless insulin pump that overcomes the physical inconveniences of conventional tubed insulin pump therapy (IPT).
Objective: We compared treatment satisfaction with Omnipod DASH System to usual care (multiple daily injections [MDIs] or tubed IPT) in adults with type 1 diabetes using self-monitoring blood glucose (SMBG).
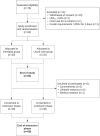
Methods: Adults with type 1 diabetes on MDI (n = 40) or IPT (n = 25) from 4 diabetes centers in Australia were randomly assigned in a 1:1 nonblinded manner to Omnipod DASH System (Omnipod group) or continue usual care (Usual Care group) for 12 weeks, followed by a further 12-week extension during which all participants used the device. The primary outcome was treatment satisfaction assessed by change in Diabetes Technology Questionnaire "current" (ΔDTQ-current) score at 12 weeks (study end). Secondary outcomes included ΔDTQ-current following extension and other participant-reported outcomes (PROs) measuring quality of life, burden of disease treatment, and glycemic and device-related outcomes at 12 weeks (study end) and 24 weeks (end extension).
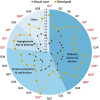
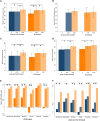
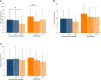
Results: Treatment satisfaction improved more in the Omnipod group vs the Usual Care group (ΔDTQ-current score of 16.4 [21.2] vs 0.0 [12.8]; P < .001) at study end. Significantly greater improvements in other PROs and glycated hemoglobin A1c were also observed. Improvements in DTQ-current and other PROs comparing study end and end extension were similar. While percentage in time in range change from baseline did not differ at study end (-2.0 [12.7] %), it was significantly greater at end extension (5.6 [10.9] %; P = .016).
Conclusion: The Omnipod DASH System resulted in greater treatment satisfaction at 12 weeks in adults with type 1 diabetes using SMBG that was sustained after 24 weeks of device use without compromising sleep quality and fear of hypoglycemia. Improvements in glycemia were also observed.
Keywords: Omnipod DASH system; quality of life; treatment satisfaction; tubeless insulin pump therapy.
© The Author(s) 2024. Published by Oxford University Press on behalf of the Endocrine Society.
Figures


Similar articles
-
A pilot randomised controlled parallel arm trial evaluating treatment satisfaction with the Omnipod DASH® Insulin Management System compared with usual care in adults with type 1 diabetes in Australia: rationale, study design and methodologies.Pilot Feasibility Stud. 2023 Oct 9;9(1):171. doi: 10.1186/s40814-023-01400-4. Pilot Feasibility Stud. 2023. PMID: 37814352 Free PMC article.
-
"You can hide it if you want to, you can let it be seen if you want to": A qualitative study of the lived experiences of Australian adults with type 1 diabetes using the Omnipod DASH® system.Diabetes Res Clin Pract. 2024 Feb;208:111123. doi: 10.1016/j.diabres.2024.111123. Epub 2024 Feb 2. Diabetes Res Clin Pract. 2024. PMID: 38309532
-
Patient perceptions of using the OmniPod system compared with conventional insulin pumps in young adults with type 1 diabetes.Diabetes Technol Ther. 2012 May;14(5):411-7. doi: 10.1089/dia.2011.0228. Epub 2012 Jan 27. Diabetes Technol Ther. 2012. PMID: 22283640 Clinical Trial.
-
Novel Bluetooth-Enabled Tubeless Insulin Pump: Innovating Pump Therapy for Patients in the Digital Age.J Diabetes Sci Technol. 2019 Jan;13(1):20-26. doi: 10.1177/1932296818798836. Epub 2018 Sep 21. J Diabetes Sci Technol. 2019. PMID: 30239214 Free PMC article. Review.
-
Optimizing type 1 diabetes after multiple daily injections and capillary blood monitoring: Pump or sensor first? A meta-analysis using pooled differences in outcome measures.Diabetes Obes Metab. 2021 Nov;23(11):2521-2528. doi: 10.1111/dom.14498. Epub 2021 Aug 12. Diabetes Obes Metab. 2021. PMID: 34286892
References
-
- Jeitler K, Horvath K, Berghold A, et al. Continuous subcutaneous insulin infusion versus multiple daily insulin injections in patients with diabetes mellitus: systematic review and meta-analysis. Diabetologia. 2008;51(6):941‐951. - PubMed
-
- Pala L, Dicembrini I, Mannucci E. Continuous subcutaneous insulin infusion vs modern multiple injection regimens in type 1 diabetes: an updated meta-analysis of randomized clinical trials. Acta Diabetol. 2019;56(9):973‐980. - PubMed
-
- Carroll MF, Schade DS. The Dawn phenomenon revisited: implications for diabetes therapy. Endocr Pract. 2005;11(1):55‐64. - PubMed
-
- Lu JC, Vogrin S, McAuley SA, et al. Meal-time glycaemia in adults with type 1 diabetes using multiple daily injections vs insulin pump therapy following carbohydrate-counting education and bolus calculator provision. Diabetes Res Clin Pract. 2021;179:109000. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

