Large Unilamellar Vesicles of Phosphatidic Acid Reduce the Toxicity of α-Synuclein Fibrils
- PMID: 38373398
- PMCID: PMC10915799
- DOI: 10.1021/acs.molpharmaceut.3c01012
Large Unilamellar Vesicles of Phosphatidic Acid Reduce the Toxicity of α-Synuclein Fibrils
Abstract
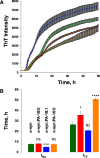
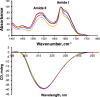
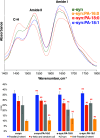
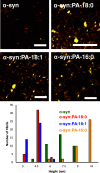
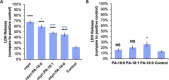
Parkinson's disease (PD) is a severe pathology that is caused by a progressive degeneration of dopaminergic neurons in substantia nigra pars compacta as well as other areas in the brain. These neurodegeneration processes are linked to the abrupt aggregation of α-synuclein (α-syn), a small protein that is abundant at presynaptic nerve termini, where it regulates cell vesicle trafficking. Due to the direct interactions of α-syn with cell membranes, a substantial amount of work was done over the past decade to understand the role of lipids in α-syn aggregation. However, the role of phosphatidic acid (PA), a negatively charged phospholipid with a small polar head, remains unclear. In this study, we examined the effect of PA large unilamellar vesicles (LUVs) on α-syn aggregation. We found that PA LUVs with 16:0, 18:0, and 18:1 FAs drastically reduced the toxicity of α-syn fibrils if were present in a 1:1 molar ratio with the protein. Our results also showed that the presence of these vehicles changed the rate of α-syn aggregation and altered the morphology and secondary structure of α-syn fibrils. These results indicate that PA LUVs can be used as a potential therapeutic strategy to reduce the toxicity of α-syn fibrils formed upon PD.
Keywords: AFM-IR; LDH; fibrils; phosphatidic acid; α-synuclein.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
References
-
- Marizzoni M.; Cattaneo A.; Mirabelli P.; Festari C.; Lopizzo N.; Nicolosi V.; Mombelli E.; Mazzelli M.; Luongo D.; Naviglio D.; et al. Short-Chain Fatty Acids and Lipopolysaccharide as Mediators Between Gut Dysbiosis and Amyloid Pathology in Alzheimer’s Disease. J. Alzheimer’s Dis. 2020, 78 (2), 683–697. 10.3233/JAD-200306. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

