High levels of fatty acid-binding protein 5 excessively enhances fatty acid synthesis and proliferation of granulosa cells in polycystic ovary syndrome
- PMID: 38373971
- PMCID: PMC10875862
- DOI: 10.1186/s13048-024-01368-6
High levels of fatty acid-binding protein 5 excessively enhances fatty acid synthesis and proliferation of granulosa cells in polycystic ovary syndrome
Retraction in
-
Retraction Note: High levels of fatty acid-binding protein 5 excessively enhances fatty acid synthesis and proliferation of granulosa cells in polycystic ovary syndrome.J Ovarian Res. 2025 Jun 16;18(1):132. doi: 10.1186/s13048-025-01713-3. J Ovarian Res. 2025. PMID: 40524211 Free PMC article. No abstract available.
Abstract
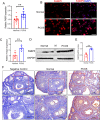
Background: Polycystic ovary syndrome (PCOS) is one of the most complex endocrine disorders in women of reproductive age. Abnormal proliferation of granulosa cells (GCs) is an important cause of PCOS. This study aimed to explore the role of fatty acid-binding protein 5 (FABP5) in granulosa cell (GC) proliferation in polycystic ovary syndrome (PCOS) patients.
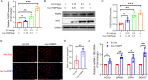
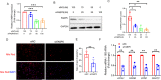
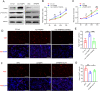
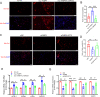
Methods: The FABP5 gene, which is related to lipid metabolism, was identified through data analysis of the gene expression profiles of GSE138518 from the Gene Expression Omnibus (GEO) database. The expression levels of FABP5 were measured by quantitative real-time PCR (qRT‒PCR) and western blotting. Cell proliferation was evaluated with a cell counting kit-8 (CCK-8) assay. Western blotting was used to assess the expression of the proliferation marker PCNA, and immunofluorescence microscopy was used to detect Ki67 expression. Moreover, lipid droplet formation was detected with Nile red staining, and qRT‒PCR was used to analyze fatty acid storage-related gene expression.
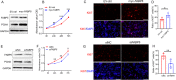
Results: We found that FABP5 was upregulated in ovarian GCs obtained from PCOS patients and PCOS mice. FABP5 knockdown suppressed lipid droplet formation and proliferation in a human granulosa-like tumor cell line (KGN), whereas FABP5 overexpression significantly enhanced lipid droplet formation and KGN cell proliferation. Moreover, we determined that FABP5 knockdown inhibited PI3K-AKT signaling by suppressing AKT phosphorylation and that FABP5 overexpression activated PI3K-AKT signaling by facilitating AKT phosphorylation. Finally, we used the PI3K-AKT signaling pathway inhibitor LY294002 and found that the facilitation of KGN cell proliferation and lipid droplet formation induced by FABP5 overexpression was inhibited. In contrast, the PI3K-AKT signaling pathway agonist SC79 significantly rescued the suppression of KGN cell proliferation and lipid droplet formation caused by FABP5 knockdown.
Conclusions: FABP5 promotes active fatty acid synthesis and excessive proliferation of GCs by activating PI3K-AKT signaling, suggesting that abnormally high expression of FABP5 in GCs may be a novel biomarker or a research target for PCOS treatment.
Keywords: FABP5; Fatty acid synthesis; Granulosa cell; PCOS; PI3K-AKT; Proliferation.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

References
-
- March WA, Moore VM, Willson KJ, Phillips DI, Norman RJ, Davies MJ. The prevalence of polycystic ovary syndrome in a community sample assessed under contrasting diagnostic criteria. Human Reprod. 2010;25(2):544–551. - PubMed
-
- Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS) Human Reprod. 2004;19(1):41–47. - PubMed
-
- Rimon-Dahari N, Yerushalmi-Heinemann L, Alyagor L, Dekel N. Ovarian Folliculogenesis. Results Probl Cell Differ. 2016;58:167–190. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

