A neurophysiological basis for aperiodic EEG and the background spectral trend
- PMID: 38374047
- PMCID: PMC10876973
- DOI: 10.1038/s41467-024-45922-8
A neurophysiological basis for aperiodic EEG and the background spectral trend
Abstract
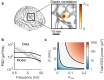
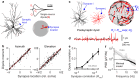
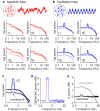
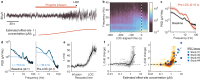
Electroencephalograms (EEGs) display a mixture of rhythmic and broadband fluctuations, the latter manifesting as an apparent 1/f spectral trend. While network oscillations are known to generate rhythmic EEG, the neural basis of broadband EEG remains unexplained. Here, we use biophysical modelling to show that aperiodic neural activity can generate detectable scalp potentials and shape broadband EEG features, but that these aperiodic signals do not significantly perturb brain rhythm quantification. Further model analysis demonstrated that rhythmic EEG signals are profoundly corrupted by shifts in synapse properties. To examine this scenario, we recorded EEGs of human subjects being administered propofol, a general anesthetic and GABA receptor agonist. Drug administration caused broadband EEG changes that quantitatively matched propofol's known effects on GABA receptors. We used our model to correct for these confounding broadband changes, which revealed that delta power, uniquely, increased within seconds of individuals losing consciousness. Altogether, this work details how EEG signals are shaped by neurophysiological factors other than brain rhythms and elucidates how these signals can undermine traditional EEG interpretation.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




References
-
- Berger H. Über das elektrenkephalogramm des menschen. Arch. Psychiatr. Nervenkr. 1929;87:527–570. doi: 10.1007/BF01797193. - DOI
-
- Steriade M. Cellular substrates of brain rhythms. Electroencephalogr. Basic Princ. Clin. Appl. Relat. fields. 2005;5:31–83.
-
- Nunez, P. L. & Srinivasan, R. Electric Fields of the Brain (Oxford Univ. Press, Oxford, 2006). 10.1093/acprof:oso/9780195050387.001.0001.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

