Multiple roles for the cytoplasmic C-terminal domains of the yeast cell surface receptors Rgt2 and Snf3 in glucose sensing and signaling
- PMID: 38374219
- PMCID: PMC10876965
- DOI: 10.1038/s41598-024-54628-2
Multiple roles for the cytoplasmic C-terminal domains of the yeast cell surface receptors Rgt2 and Snf3 in glucose sensing and signaling
Abstract
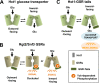
The plasma membrane proteins Rgt2 and Snf3 are glucose sensing receptors (GSRs) that generate an intracellular signal for the induction of gene expression in response to high and low extracellular glucose concentrations, respectively. The GSRs consist of a 12-transmembrane glucose recognition domain and a cytoplasmic C-terminal signaling tail. The GSR tails are dissimilar in length and sequence, but their distinct roles in glucose signal transduction are poorly understood. Here, we show that swapping the tails between Rgt2 and Snf3 does not alter the signaling activity of the GSRs, so long as their tails are phosphorylated in a Yck-dependent manner. Attachment of the GSR tails to Hxt1 converts the transporter into a glucose receptor; however, the tails attached to Hxt1 are not phosphorylated by the Ycks, resulting in only partial signaling. Moreover, in response to non-fermentable carbon substrates, Rgt2 and Hxt1-RT (RT, Rgt2-tail) are efficiently endocytosed, whereas Snf3 and Hxt1-ST (ST, Snf3-tail) are endocytosis-impaired. Thus, the tails are important regulatory domains required for the endocytosis of the Rgt2 and Snf3 glucose sensing receptors triggered by different cellular stimuli. Taken together, these results suggest multiple roles for the tail domains in GSR-mediated glucose sensing and signaling.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures





References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

