Cooperative control of a DNA origami force sensor
- PMID: 38374280
- PMCID: PMC10876929
- DOI: 10.1038/s41598-024-53841-3
Cooperative control of a DNA origami force sensor
Abstract
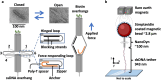
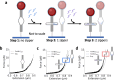
Biomolecular systems are dependent on a complex interplay of forces. Modern force spectroscopy techniques provide means of interrogating these forces, but they are not optimized for studies in constrained environments as they require attachment to micron-scale probes such as beads or cantilevers. Nanomechanical devices are a promising alternative, but this requires versatile designs that can be tuned to respond to a wide range of forces. We investigate the properties of a nanoscale force sensitive DNA origami device which is highly customizable in geometry, functionalization, and mechanical properties. The device, referred to as the NanoDyn, has a binary (open or closed) response to an applied force by undergoing a reversible structural transition. The transition force is tuned with minor alterations of 1 to 3 DNA oligonucleotides and spans tens of picoNewtons (pN). The DNA oligonucleotide design parameters also strongly influence the efficiency of resetting the initial state, with higher stability devices (≳10 pN) resetting more reliably during repeated force-loading cycles. Finally, we show the opening force is tunable in real time by adding a single DNA oligonucleotide. These results establish the potential of the NanoDyn as a versatile force sensor and provide fundamental insights into how design parameters modulate mechanical and dynamic properties.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



Update of
-
Cooperative control of a DNA origami force sensor.bioRxiv [Preprint]. 2023 Jun 28:2023.06.26.546608. doi: 10.1101/2023.06.26.546608. bioRxiv. 2023. Update in: Sci Rep. 2024 Feb 19;14(1):4132. doi: 10.1038/s41598-024-53841-3. PMID: 37425797 Free PMC article. Updated. Preprint.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

